Abstract
Purpose
Toxoplasma gondii is transmitted congenitally or acquired by consumption of food and water contaminated with cysts or oocysts. This study aimed at genotyping T. gondii strains from slaughtered goats in Jahrom.
Methods
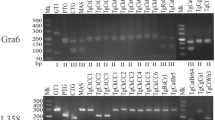
A total of 561 specimens (heart, diaphragm, and tongue) from 187 slaughtered goats were collected from Jahrom slaughterhouse. After DNA extraction, the T. gondii strains were genotyped by the nested PCR–RFLP based on GRA6 and 3ʹ, and 5ʹ ends of the SAG2 gene.
Results
T. gondii infection was present in 18.2% of cases. Among the examined organs, the diaphragm was more disposed to the infection (10.2%). Furthermore, infection rates of the heart and tongue were 8.6% and 3.7%, respectively. Concurrent infection in the heart and diaphragm, tongue and diaphragm, and heart and tongue were 3.2%, 0.5%, and 0.5%, respectively. In genotyping experiments, genotype I was the most frequent genotype of T. gondii (58.8%), followed by type II (23.5%), type III (11.8%), and a combination of type I and II (5.9%).
Conclusions
The results of this study showed the presence of different genotypes of T. gondii in goats including three major and mixed genotypes. These results can be useful in toxoplasmosis control and prevention.




Similar content being viewed by others
References
Flegr J, Prandota J, Sovičková M, Israili ZH (2014) Toxoplasmosis—a global threat. Correlation of latent Toxoplasmosis with specific disease burden in a set of 88 countries. PLoS One 9(3):90203. https://doi.org/10.1371/journal.pone.0090203
Montoya JG, Liesenfeld O (2004) Toxoplasmosis. Lancet 363:1965–1976. https://doi.org/10.1016/S0140-6736(04)16412-X
Sarkari B, Asgari Q, Bagherian N, Ashkani Esfahani S, Kalantari M, Mohammadpour I et al (2014) Molecular and serological evaluation of Toxoplasma gondii infection in reared turkeys in Fars province. Iran Jundishapur J Microbiol 7(7):e11598. https://doi.org/10.5812/jjm.11598
Asgari Q, Mohammadpour I, Pirzad R, Kalantari M, Motazedian MH, Naderi S (2018) Molecular and serological detection of Toxoplasma gondii in stray cats in Shiraz, south-central, Iran. Iran J Parasitol 13(3):430–439 (PMID: 30483335)
Weiss LM, Dubey JP (2009) Toxoplasmosis: a history of clinical observations. Int J Parasitol 39(8):895–901. https://doi.org/10.1016/j.ijpara.2009.02.004
Omidian M, Ganjkarimi AH, Asgari Q, Hatam G (2020) Molecular and serological study on congenital toxoplasmosis in newborn of Shiraz, Southern Iran. Environ Sci Pollut Res 28:1–7. https://doi.org/10.1007/s11356-020-11707-x
Arefkhah N, Sarkari B, Asgari Q, Moshfe A, Khalafi MH, Mohammadpour I (2020) Molecular genotyping of Toxoplasma gondii in sheep aborted fetuses reveals predominance of type I infection in Southwest of Iran. Iran J Parasitol 15(3):374–382. https://doi.org/10.18502/ijpa.v15i3.4202
Fuentes I, Rubio JM, Ramı́rez C, Alvar J (2001) Genotypic characterization of Toxoplasma gondii strains associated with human toxoplasmosis in Spain: direct analysis from clinical samples. J Clin Microbiol 39(4):1566–1570. https://doi.org/10.1128/JCM.39.4.1566-1570.2001
Hassan MA, Olijnik AA, Frickel EM, Saeij JP (2019) Clonal and atypical Toxoplasma strain differences in virulence vary with mouse sub-species. Int J Parasitol 49(1):63–70. https://doi.org/10.1016/j.ijpara.2018.08.007
Petersen E, Dubey J P (2001) Biology of toxoplasmosis. Clinical toxoplasmosis: prevention and management, DHM Joynson & TG Wreghitt (Eds.): 1–42. http://assets.cambridge.org/052101/9427/excerpt/0521019427_excerpt.pdf. Accessed Oct 2020
Kompalic-Cristo A, Frotta C, Suárez-Mutis M, Fernandes O, Britto C (2007) Evaluation of a real-time PCR assay based on the repetitive B1 gene for the detection of Toxoplasma gondii in human peripheral blood. Parasit Res 101(3):619–625. https://doi.org/10.1007/s00436-007-0524-9
Khan A, Noordin R (2020) Serological and molecular rapid diagnostic tests for Toxoplasma infection in humans and animals. Eur J Clin Microbiol Infect Dis 39(1):19–30. https://doi.org/10.1007/s10096-019-03680-2
Khan A, Su C, German M, Storch GA, Clifford DB, Sibley LD (2005) Genotyping of Toxoplasma gondii strains from immunocompromised patients reveals high prevalence of type I strains. J Clin Microbiol 43(12):5881–5887. https://doi.org/10.1128/JCM.43.12.5881-5887.2005
Howe DK, Honoré S, Derouin F, Sibley LD (1997) Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J Clin Microbiol 35(6):1411–1414. https://doi.org/10.1128/jcm.35.6.1411-1414.1997
Switaj K, Master A, Skrzypczak M, Zaborowski P (2005) Recent trends in molecular diagnostics for Toxoplasma gondii infections. Clin Microbiol Inf 11(3):170–176. https://doi.org/10.1111/j.1469-0691.2004.01073.x
Kalantari N, Ghaffari S, Bayani M, Agapour R, Zeinalzadeh M, Gavipanjeh F, et al (2014) Serological study of toxoplasmosis in pregnant women in Babol, northern Iran 2012–2013. Sci J Ilam Univ Med Sci 22(4): 102–8. https://sjimu.medilam.ac.ir/article-1-1287-en.html. Accessed Oct 2020
Boughattas S, Ben-Abdallah R, Siala E, Souissi O, Aoun K, Bouratbine A (2010) Direct genotypic characterization of Toxoplasma gondii strains associated with congenital toxoplasmosis in Tunisia (North Africa). Am J Trop Med Hyg 82(6):1041–1046. https://doi.org/10.4269/ajtmh.2010.09-0691
Xiao J, Prandovszky E, Kannan G, Pletnikov MV, Dickerson F, Severance EG et al (2018) Toxoplasma gondii: biological parameters of the connection to schizophrenia. Schizophr Bull 44(5):983–992. https://doi.org/10.1093/schbul/sby082
Miao Q, Huang S-Y, Qin S-Y, Yu X, Yang Y, Yang J-F et al (2015) Genetic characterization of Toxoplasma gondii in Yunnan black goats (Capra hircus) in southwest China by PCR-RFLP. Parasit Vectors 8(1):57. https://doi.org/10.1186/s13071-015-0673-0
Satbige AS, Bharathi MV, Ganesan P, Sreekumar C, Rajendran C (2016) Detection of Toxoplasma gondii in small ruminants in Chennai using PCR and modified direct agglutination test. J Parasit Dis 40(4):1466–1469. https://doi.org/10.1007/s12639-015-0713-x
Lopes AP, Vilares A, Francisco N, Rodrigues A, Martins T, Ferreira I et al (2015) Genotyping characterization of Toxoplasma gondii in cattle, sheep, goats and swine from the north of Portugal. Iran J Parasitol 10(3):465 (PMID: 26622302)
Asgari Q, Sarnevesht J, Kalantari M, Sadat SJA, Motazedian MH, Sarkari B (2011) Molecular survey of Toxoplasma infection in sheep and goat from Fars province, Southern Iran. Trop Anim Health Product 43(2):389–392. https://doi.org/10.1007/s11250-010-9704-1
Aliabadi J, Ziaali PN (2016) Survey of Toxoplasma gondii in livestocks' meat (Sheep, Goat, Camel), using nested PCR method in Sabzavar district. Europ Online J Nat Soc Sci 5(2): 368. https://european-science.com/eojnss/article/view/4611. Accessed Oct 2020
Foroghi Borj H (2018) Survey on frequency of toxoplasma gondii in livestock meat (sheep, goats and cattle) by using serology & PCR methods in Quchan city. Master's thesis, School of Medicine, Kerman University of Medical Sciences, Kerman, Iran. http://eprints.kmu.ac.ir/27085/1/6485_20180408_0001.pdf. Accessed Oct 2020
Acknowledgements
The authors wish to thank Vice Canceller of Jahrom University of Medical Sciences for its financial support to accomplish this research project.
Funding
This study was supported by the Deputy of Jahrom University of Medical Sciences for its financial support to implement the project (IR.JUMS.REC.1396.097) awarded to the Hassan Rezanezhad.
Author information
Authors and Affiliations
Contributions
RS, HR, and MK conducted experiments, writing, and editing the figures of the first draft. HR, KS, and SE performed molecular biology and experimental setup to produce the particles. MK, BP, BA, and MEJ contributed to the design, analysis, and manuscript preparation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Ethics Approval
This study was approved by the Jahrom University of Medical Sciences Ethics Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sadooni, R., Rezanezhad, H., Solhjoo, K. et al. Genotyping of Toxoplasma gondii Strains from Goats in Jahrom District, Southern Iran. Acta Parasit. 67, 454–459 (2022). https://doi.org/10.1007/s11686-021-00481-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-021-00481-6