Abstract
Purpose
Predatory fungi have been the subject of fundamental studies and their potential as biological control agents against parasitic plant nematodes has been assessed. The aim of the present study was to isolate and identify predatory fungi, performing in vitro and in vivo screening to select highly active strains to control parasitic nematodes.
Methods
Different nutrient media were used to isolate predatory fungi and determine their morphological and cultural properties. Identification was performed by classical and molecular biology methods. In vitro and in vivo screening was conducted to select highly active strains.
Results
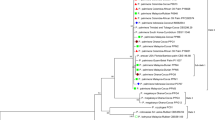
Twelve isolates of Arthrobotrys oligospora (Orbiliomycetes) found in nature were investigated for their predaceous efficacy against garlic stem nematodes (Ditylenchus dipsaci). The effect of temperature and pH on the growth rate and trap formation of representative isolates was determined and isolates were characterized by light microscopy and molecular markers. BLAST was used to sequence the rDNA internal transcribed spacer of A. oligospora isolate KTMU-7. The optimum growth of A. oligospora strains was achieved at 20–25 °C on 1–2% corn meal agar (CMA) within the pH range of 5.6–8.6. The factors responsible for the trap formation of these fungal strains were identified. In vitro and in vivo experiments were performed to evaluate the nematicidal activity of local predatory fungal isolates against soil nematodes.
Conclusions
Preliminary studies proved A. oligospora to be a potentially effective biological control agent, immobilizing 85.7 ± 2.19% of garlic stem nematodes in soil from the rhizosphere of potato plants.
















Similar content being viewed by others
References
Barron GL (1977) The nematode-destroying fungi. In: Topics in mycobiology 1. Canada, Canadian Biological Publications Ltd. https://doi.org/10.1002/jobm.19790190412
Liou GY, Tzean SS (1997) Phylogeny of the genus Arthrobotrys and allied nematode-trapping fungi based on rDNA sequences. Mycologia 89:876–884. https://doi.org/10.2307/3761108
Yang Y, Yang E, An Z, Liu X (2007) Evolution of nematode-trapping cells of predatory fungi of the Orbiliaceae based on evidence from rRNA-encoding DNA and multiprotein sequences. PNAS 104:8379–8384. https://doi.org/10.1073/pnas.0702770104
Dong LQ, Zhang KQ (2006) Microbial control of plant-parasitic nematodes: a five-party interaction. Plant Soil 288:31–45
Van OC (2011) Fungal pathogenesis: hungry fungus eats nematode. Nat Rev Microbiol 9:766–767
Ojeda-Robertos NF, Torres-Acosta JFJ, Ayala-Burgos AJ, Sandoval- Castro CA, Valero Coss RO, Mendoza-de-Gives P (2009) Digestibility of Duddingtonia flagrans chlamydospores in ruminants: in vitro and in vivo studies. BMC Vet Res 107:103–108
Paz-Silva A, Francisco I, Valero-Coss RO, Cortiñas FJ, Sánchez JA, Francisco R, Gives PM (2011) Ability of the fungus Duddingtonia flagrans to adapt to the cyathostomin egg-output by spreading chlamydospores. Vet Parasitol 30:277–282
Zopf W (1888) Zur Kenntnis der Infektionskrankheiten niederer Thiere und Pflanzen. Nova Acad Caes Leop German Nat Cur 52:314–376
Friman E, Olsson S, Nordbring Hertz B (1985) Heavy trap formation by Arthrobotrys oligospora in liquid culture. FEMS Microbiol Ecol 31:17–20
Veenhuis M, Nordbring-Hertz B, Harder W (1985) An electron microscopical analysis of capture and initial stages of penetration of nematodes by Arthrobotrys oligospora. Antonie Van Leeuwenhoek 51:385–398
Swe A, Jeewon R, Pointing SB, Hyde KD (2009) Diversity and abundance of nematode trapping fungi from decaying litter in terrestrial, freshwater and mangrove habitats. Biodivers Conserv 18:1695–1714
Wang FH, Wang BB, Xu CL, Cai KZ (2015) Influence of temperature and pH value on the growth of Arthrobotrys oligospora and its biological characteristics observation. J Gansu Agric Univ 50:100–105
Jaffee BA (2004) Wood, nematodes, and the nematode-trapping fungus Arthrobotrys oligospora. Soil Biol Biochem 36:1171–1178
Jansson HB, Lopez-Llorca LV (2004) Control of nematodes by fungi. In: Arora DK (ed) Fungal biotechnology in agricultural, food, and environmental applications. Marcel Dekker, New York
Lee JK, Kim DG, Lee SB (2004) Nutritional requirements and mass production of nematode-trapping fungus, Arthrobotrys oligospora. J Asia Pac Entomol 7:325–329
Singh RK, Kumar N, Singh KP (2005) Morphological variations in Arthrobotrys oligospora on different media. Mycobiology 33(2):118–120
Nordbring-Hertz B (2004) Morphogenesis in the nematode trapping fungus Arthrobotrys oligospora an extensive plasticity of infection structures. Mycologist 18:125–133
Migunova VD, Byzov BA (2005) Determinants of trophic modes of the nematophagous fungus Arthrobotrys oligospora interacting with bacterivorous nematode Caenorhabditis elegans. Pedobiologia 49:101–108
Pramer D, Stoll NR (1959) Nemin: a morphogenic substance causing trap formation by predaceous fungi. Science 129:966–967. https://doi.org/10.1126/science.129.3354.966
Bargmann CI, Horvitz HR (1991) Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7:729–742. https://doi.org/10.1016/0896-6273(91)90276-6
Bargmann CI (2006) Chemosensation in C. elegans. WormBook, ed. The C. elegans Research Community, WormBook. https://doi.org/10.1895/wormbook.1.123.1
Hsueh YP, Mahanti P, Schroeder FC, Sternberg PW (2013) Nematode-trapping fungi eavesdrop on nematode pheromones. Curr Biol 23:83–86. https://doi.org/10.1016/j.cub.2012.11.035
Hsueh YP, Gronquist MR, Schwarz EM, Nath RD, Lee CH, Gharib S, Schroeder FC, Schroeder FC (2017) Nematophagous fungus Arthrobotrys oligospora mimics olfactory cues of sex and food to lure its nematode prey. Genom Evolut Biol Neurosci 1:21. https://doi.org/10.7554/eLife
Nguyen VL, Bastow JL, Jaffee BA, Strong DR (2007) Response of nematode-trapping fungi to organic substrates in a coastal grassland soil. Mycol Res 111:856–862
Hoffmeister D, Keller NP (2007) Natural products of filamentous fungi: enzymes, genes, and their regulation. Nat Prod Rep 24:393–416
Jaffee BA, Strong DR (2005) Strong bottom-up and weak top-down effect in soil: nematode-parasitized insects and nematode-trapping fungi. Soil Biol Biochem 37:1011–1021
Araújo JV, Mota MA, Campos AK (2004) Controle biológico de helmintos parasitos de animais por fungos nematófagos. Rev Bras Parasitol Vet 13:165–170
Stirling GR (1991) Biological control of plant-parasitic nematodes: progress, problems and prospects. CAB International, Wallingford
Ciancio A, Mukerji KG (2008) Integrated management and biocontrol of vegetable and grain crops nematodes. Springer, Dordrecht
Jatala P (1986) Biological control of plant-parasitic nematodes. Annu Rev Phytopathol 24:453–489
Liu XF, Zhang KQ (2003) Dactylella shizishanna sp. nov., from Shizi Mountain, China. Fungal Divers 14:103–107
Hao Y, Mo M, Su H, Zhang KQ (2005) Ecology of aquatic nematode-trapping hyphomycetes in southwestern China. Aquat Microb Ecol 40:175–181. https://doi.org/10.3354/ame040175
Van OC (1985) Taxonomy of the Dactylaria complex. V. A review of Arthrobotrys and allied genera. Stud Mycol 26:61–96
Schenick S, Kendrick WB, Pramer D (1977) A new nematode—trapping hyphomycetes and a revaluation of Dactylaria and Arthrobotrys. Can J Bot 55:977–985
Li TF, Zhang KQ, Liu XZ (2000) Taxonomy of nematophagous fungi. Chinese Scientific and Technological Publications, Beijing
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol 16(12):1799–1808
Liu XZ, Xiang MC, Che YS (2009) The living strategy of nematophagous fungi. Mycoscience 50:20–25
Wang F-H, Qiang Xu, Wang B-B, Wang K-Y, Xue Y-J, Cai B, Wang F, Liu Y-Q, Cai K-Z, Cao X (2017) Isolation, Identification and characterisation of the nematophagous fungus Arthrobotrys thaumasia (Monacrosporium thaumasium) from China. Biocontrol Sci Technol 27(3):378–392. https://doi.org/10.1080/09583157.2017.1291908
Mai WF, Mullin PG (1996) Plant parasitic nematode. A pictorial key to genera, 5th edn. Cornell University Press, Ithaca
Maggenti AR (1991) General nematode morphology. In: Nickel WR (ed) Manual of agricultural nematology. Marcel Dekker, New York, pp 1–46
Kepenekci İ (2012) Nematology (Plant parasite and entomopathogenic nematodes) [general nematology (Volume-I), taxonomic nematology (Volume-II). Agricultural Science Series Publication, Ankara
Southey JF (1989) Laboratory methods for work with plant and soil nematodes. 6th edn., HMSO
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comput Biol 7:203–214
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Drechsler C (1937) Some hyphomycetes that prey on free living terricolous nematodes. Mycologia 29:447–552
Haard K (1968) Taxonomic studies on the genus Arthrobotrys corda. Mycologia 60:1140–1159
Jansson H-B, Norbring-Hertz B (1979) Attraction of nematodes to living mycelium of nematophagous fungi. J Gen Microbiol 112:89–93
Jansson H-B (1982) Attraction of nematodes to endoparasitic nematophagous fungi. Trans Br Mycol Soc 79:25–29
Jansson H-B (1982) Predacity by nematophagous fungi and its relation to the attraction of nematodes. Microb Ecol 8:233–240
Bouwman LA, Van Den Boogert BJ, Breme PHJF, Hoenderboom GHJ, De Ruilter PC (1994) Short-term and long term effects of bacterivorous nematodes and nematophagous fungi on carbon and nitrogen mineralisation in microcosm. Biol Fertil Soils 17:249–256
Duponnois R, Mateille T, Gueye M (1995) Biological characteristics and effects of two strains of Arthrobotrys oligospora from Senegal on Meloidogyne species (with reference to M. mayaguensis) parasitizing tomato plants. Biocontrol Sci Technol 5:517–525
Gomes APS, Vasconcellos RS, Ramos ML, Guimaraes MP, Yatsuda AP, Vieira-Bressan MCR (2001) In vitro interaction of Brazilian strains of the nematode-trapping fungi Arthrobotrys spp. on Panagrellus sp. and Cooperia punctata. Mem Inst Oswaldo Cruz Rio de Janeiro 96:861–864
Galper S, Eden LM, Strling GR, Smith LJ (1995) Simple screening methods for assessing the predacious activity of nematode trapping fungi. Nematologica 41:130–140
Acknowledgements
The authors gratefully acknowledge receipt of financial support from the German Ministry of Education and Research (BMBF) research grant under funding ID 01DK17004 (research project BioControl). Saikal Bobushova has been granted a scholarship by the German Academic Exchange Service (DAAD, Grant number 91692517).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Doolotkeldieva, T., Bobushova, S., Muratbekova, A. et al. Isolation, Identification, and Characterization of the Nematophagous Fungus Arthrobotrys oligospora from Kyrgyzstan. Acta Parasit. 66, 1349–1365 (2021). https://doi.org/10.1007/s11686-021-00404-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-021-00404-5