Abstract
Introduction
Cryptosporidiosis is an opportunistic globally distributed parasitic disease caused by protozoan Cryptosporidium where its development is closely related to the host’s immune status. New therapeutic agents are a high priority as chemotherapeutics are impractical and vaccines are unavailable for young as well as immune-compromised patients or animals. The current study was designed to evaluate the therapeutic effect of the internal white (albedo) and external yellow (flavedo) peels of Citrus maxima (C. maxima) as an alternative medicinal plant.
Materials and methods
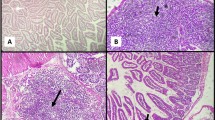
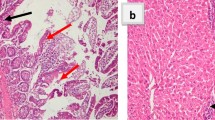
Parasitological examination for oocysts in the stool was determined. Histopathological alterations and immunohistochemical expression of APC and cyclin D1 as well as an assessment of interferon-γ (IFN-γ) and interleukin 1β (IL-1β) in ileal tissues was carried out. In addition, the biochemical examination of serum albumin, globulin and liver enzymes were evaluated. Results revealed a significant decrease of oocysts count correlated with an amelioration of histopathological and immunohistochemical changes in ileal tissue with an enhancement of liver enzymes and inflammatory cytokines levels.
Conclusion
It could be concluded that treatment with C. maxima peel extracts have a potential therapeutic and an immunoregulatory efficacy against Cryptosporidiosis. Obtained results showed that the white peel was found to have more immunological effect that could significantly enhance inflammatory cytokines response towards normal status. Hence, it can be used in the daily animal diet to give protective effects against infection.



















Similar content being viewed by others
References
Aboelsoued D, Shaapan RM, Ekhateeb RMM, El-Nattat WS, Fayed AHM, Hammam AMM (2020) Therapeutic efficacy of ginger (Zingiber officinale), ginseng (Panax ginseng) and sage (Salvia officinalis) against Cryptosporidium parvum in experimentally infected mice. Egypt J Vet Sci 51(2):241–251. https://doi.org/10.21608/ejvs.2020.24183.1152
Jin Z, Ma J, Zhu G, Zhang H (2019) Discovery of novel anti-cryptosporidial activities from natural products by in vitro high-throughput phenotypic screening. Front Microbiol 10:1999. https://doi.org/10.3389/fmicb.2019.01999
Azeez SS, Alsakee HM (2017) Cryptosporidium spp. and rotavirus gastroenteritis and change of incidence after rotavirus vaccination among children in Raparin Pediatrics Hospital. Erbil Med J Indones 26:190–197. https://doi.org/10.13181/mji.v26i3.1957
Widmer G, Carmena D, Kváč M, Chalmers RM, Kissinger JC, Xiao L, Sateriale A, Striepen B, Laurent F, Lacroix-Lamandé S, Gargala G, Favennec L (2020) Update on Cryptosporidium spp.: highlights from the seventh international Giardia and Cryptosporidium conference. Parasite 27:14. https://doi.org/10.1051/parasite/2020011
Laurent F, Lamandé SL (2017) Innate immune responses play a key role in controlling infection of the intestinal epithelium by Cryptosporidium. Int J Parasitol 47:711–721. https://doi.org/10.1016/j.ijpara.2017.08.001
Ivanova DL, Denton SL, Kevin D, Fettel KD, Sondgeroth KS, Gutierrez JM, Bangoura B, Dunay IR, Gigley JP (2019) Innate lymphoid cells in protection, pathology, and adaptive immunity during Apicomplexan infection. Front Immunol 10:196. https://doi.org/10.3389/fimmu.2019.00196
Ehikgiator HN, McNair N, Mead JR (2007) Cryptosporidium parvum: the contribution of Th1-inducing pathways to the resolution of infection in mice. Exp Parasitol 115:107–113. https://doi.org/10.1016/j.exppara.2006.07.001
Seo GY, Giles DA, Kronenberg M (2020) The role of innate lymphoid cells in response to microbes at mucosal surfaces. Mucosal Immunol 13:399–412. https://doi.org/10.1038/s41385-020-0265-y
Gookin JL, Chiang S, Allen J, Armstrong MU, Stauffer SH, Finnegan C, Murtaug MP (2006) NF-B-mediated expression of iNOS promotes epithelial defense against infection by Cryptosporidium parvumin neonatal piglets. Am J Physiol Gastrointest Liver Physiol 290(1):164–174. https://doi.org/10.1152/ajpgi.00460.2004
Certad G, Ngouanesavanh T, Guyot K, Gantois N, Chassat T, Mouray A et al (2007) Cryptosporidium parvum, a potential cause of colic adenocarcinoma. Infect Agent Cancer 2:22. https://doi.org/10.1186/1750-9378-2-22
Osman M, Benamrouzz S, Guyot k, Baydoun M, Frealle e, Chabe M et al (2017) High association of Cryptosporidium spp. infection with colon adenocarcinoma in Lebanese patients. PLoS ONE 12(12):e0189422
Sulżyc-Bielicka V, Kołodziejczyk L, Jaczewska S, Bielicki D, Safranow K, Bielicki P, Kładny J, Rogowski W (2018) Colorectal cancer and Cryptosporidium spp. Infect PLoS One 13(4):e0195834. https://doi.org/10.1371/journal.pone.0195834
Zhang N, Yu X, Zhang H, Cui L, Li X, Zhang X, Gong P, Li J, Li Z, Wang X et al (2020) Prevalence and genotyping of Cryptosporidium parvumin gastrointestinal cancer patients. J Cancer 11:3334–3339. https://doi.org/10.7150/jca.42393
Cunningham D, Atkin W, Lenz HJ et al (2010) Colorectal cancer. Lancet 375:1030–1047. https://doi.org/10.1016/S0140-6736(10)60353-4
Mostafa NE, Abdel Hamid EF, Fawzy EM, Zalat RS, Rashed HE, Mohamed SY (2018) The new trend in the treatment of experimental cryptosporidiosis and the resulting intestinal dysplasia. Colorect cancer. 7(4):CRC06. https://doi.org/10.2217/crc-2018-0008
Albasri AM, Elkablawy MA, Ansari IA, Alhujaily AS (2019) Prognostic significance of cyclin D1 over-expression in colorectal cancer: an experience from Madinah, Saudi Arabia. Asian Pac J Cancer Prev 20(8):2471–2476. https://doi.org/10.31557/APJCP.2019.20.8.2471
Zhang L, Shay JW (2017) Multiple roles of APC and its therapeutic implicationsin colorectal cancer. JNCI J Natl Cancer Inst 109(8):djw332. https://doi.org/10.1093/jnci/djw332
Chavez MA, White AC (2018) Novel treatment strategies and drugs in development for cryptosporidiosis. Expert Rev Anti Infect Ther 16:655–661. https://doi.org/10.1080/14787210.2018.1500457
Agnamey P, Djeddi D, Diallo A et al (2010) Childhood cryptosporidiosis: a case report. J Parasitol Res. https://doi.org/10.1155/2010/j
Gargala G (2008) Drug treatment and novel drug target against Cryptosporidium. Parasite 15:275–281. https://doi.org/10.1051/parasite/2008153275
Singh A, Mishra A, Chaudhary R, Kumar V (2020) Dear role of herbal plants in prevention and treatment of parasitic diseases. J Sci Res 64(1):50–58. https://doi.org/10.37398/JSR.2020.640106
Abdel Megeed KN, Hammam AM, Morsy GH, Khalil FAM, Seliem MME, Aboelsoued D (2015) Control of cryptosporidiosis in buffalo calves using garlic (Allium sativum) and Nitazoxanide with special reference to some biochemical parameters. Glob Vet 14(5):646–655. https://doi.org/10.5829/idosi.gv.2015.14.05.94137
Abu El Ezz NMT, Khalil AM, Shaapan RM (2011) Therapeutic effect of onion (Allium cepa) and cinnamon (Cinnamomum zeylanicum) oils on Cryptosporidiosis in experimentally infected mice. Glob Vet 7(2):179–183
Al-Mathal EM, Alsalem AA (2013) Pomegranate (Punica granatum) peel is effective in a murine model of experimental Cryptosporidium parvum ultrastructural studies of the ileum. Exp Parasitol 134:482–494. https://doi.org/10.1016/j.exppara.2013.05.004
Feng WW, Kuang SY, Tu C, Ma ZJ, Pang JY, Wang YH, Zang Q, Liu T, Zhao Y, Xiao Y, Wang J (2018) Natural products berberine and curcumin exhibited better ameliorative effects on rats with non-alcohol fatty liver disease than lovastatin. Biomed Pharmacother 99:325–333. https://doi.org/10.1016/j.biopha.2018.01.071
Tocmo R, Pena-Fronteras J, Calumba KF, Melanie Mendoza M, Johnson JJ (2020) Valorization of pomelo (Citrus grandis Osbeck) peel: a review of current utilization, phytochemistry, bioactivities, and mechanisms of action. Compr Rev Food Sci Food Saf. https://doi.org/10.1111/1541-4337.12561
Ali G, Hawa ZEJ (2010) Synthesis of phenolics and flavonoids in ginger (Zingiber officinale Roscoe) and their effects on photosynthesis rate. Asmah R. Int J Mol Sci 11:4539–4555. https://doi.org/10.3390/ijms11114539
Liu Q, Lu L, Xião M (2012) Cell surface engineering of α-l-rhamnosidase for naringin hydrolysis. Bioresour Technol 123:144–149. https://doi.org/10.1016/j.biortech.2012.05.083
Shivananda A, Muralidhara RD, Jayaveera KN (2013) Analgesic and anti-inflammatory activities of Citrus maxima (J. Burm) Merr. in animal models. Res J Pharma Biol Chem Sci 4(2):1800
Klangpetch W, Phromsurin K, Hannarong K, Wichaphon J, Rungchang S (2016) Antibacterial and antioxidant effects of tropical Citrus peel extracts to improve the shelf life of raw chicken drumettes. Int Food Res J 23(2):700–707
Toh JJ, Khoo HE, Azrina A (2013) Comparison of antioxidant properties of pomelo [Citrusgrandis (L) Osbeck] varieties. Int Food Res J 20(4):1661–1668.
Ahmed WFA, Bahnasy RM, Zedan AMG (2015) Parasitological and biochemical parameters in Schistosoma mansoni infected mice and treated with aqueous thymus leaves and Citrus maxima (pomelo) peels extracts. J Am Sci 11(10):95–103
Reshmi SK, Sudha ML, Shashirekha MN (2020) Noodles fortified with Citrus maxima (pomelo) fruit segments suiting the diabetic population. Bioact Carbohydr Diet Fibre 22:100213. https://doi.org/10.1016/j.bcdf.2020.100213
Mahmoud MH, Wahba HM, Mahmoud MH, Abu-Salem FM (2018) Antagonizing the hazardous impact of increased oxidative stress in Wistar rats by biscuits with dried orange peel. J Biol Sci 18(1):21–31. https://doi.org/10.3923/jbs.2018.21.31
Aboelsoued D, Abo-Aziza FAM, Mahmoud MH, Abdel Megeed KN, Abu El Ezz MNT, Abu-Salem FM (2019) Anticryptosporidial effect of pomegranate peels water extract in experimentally infected mice with special reference to some biochemical parameters and antioxidant activity. J Parasite Dis 43(2):215–228. https://doi.org/10.1007/s12639-018-01078-z
Current WL, Reese NC (1986) A comparison of endogenous development of three isolates of Cryptosporidium in suckling mice. J Protozool 33:98–108. https://doi.org/10.1111/j.1550-7408.1986.tb05567
Abdou AG, Harba NM, Afifi AF, Elnaidany NF (2013) Assessment of Cryptosporidium parvum infection in immunocompetent and immunocompromised mice and its role in triggering intestinal dysplasia. Int J Infect Dis 17:593–600. https://doi.org/10.1016/j.ijid.2012.11.023
Henriksen SA, Pohlenz JF (1981) Staining of cryptosporidia by a modified Ziehl–Neelsen technique. Acta Vet Scand 22:594–596
Cannon DC, Olitzky I, Inkpen JA (1974) Clinical chemistry principles and techniques of determination of total protein, 2nd edn. Harper and Rowpubl, London
Winn-Deen ES, David H, Sigler G, Chavez R (1988) Determination of total and pancreatic α-amylase in human serum with 2-chloro-4-nitrophenyl-α-d-maltotrioside as substrate. Clin Chem 34:2005. https://doi.org/10.1016/S0009-8981(96)06481-9
Bancroft JD, Stevens GA (1990) Theory and practice of histological techniques, 2nd edn. Churchill Livingstone, London
Hao XP, Pretlow TG, Rao JS, Pretlow TP (2002) β-Catenin expression is altered in human colon aberrant cript foci. Cancer Res 61:8085–8088
Cao S, Xu M, Jiang Y, Liu H, Yuan Z, Sun L, Cao J, Shen Y (2020) Characterization of Cryptosporidium, Giardia and Enterocytozoon in Chickens From Ezhou, Hubei. China Front Vet Sci 7:30. https://doi.org/10.3389/fvets.2020.00030
Petry F, Robinson HA, McDonald V (1995) Murine infection model for maintenance and amplification of Cryptosporidium parvum oocysts. J Clin Microbiol 33:1922–1924. https://doi.org/10.1128/JCM.33.7.1922-1924.1995
John J, Mehta A, Shukla S, Mehta P (2009) A report on anthelmintic activity of Cassia tora leaves. J Sci Technol 31(3):269–271
Abdelrahman KA, Abdel Megeed KN, Hammam AM, Morsy GH, Seliem MME, Aboelsoued D (2015) Molecular characterization of bubaline isolate of Cryptosporidium species from Egypt. Res J Parasitol 10(4):127–141
Cui Z, Song D, Qi M, Zhang S, Wang R, Jian F, Ning C, Zhang L (2018) Revisiting the infectivity and pathogenicity of Cryptosporidium avium provides new information on parasitic sites within the host. Parasites Vector 11:514. https://doi.org/10.1186/s13071-018-3088-x
Enwezor FNC, Sackey AKB (2005) Camel trypanosomosis—a review. Vet Arh 75:439–452
Sajal Gupta S, Johnson A, Meyrick S, Davies AP, Chalmers R (2018) A case of hepato-biliary infection secondary to cryptosporidium in a patient on tacrolimus. MM Case Rep. https://doi.org/10.1099/jmmcr.0.005159
Azza MK (2008) Some biochemical, hematological and clinical studies of selected ruminal and blood constituents in camels affected by various diseases. Res J Vet Sci 1(1):16–27. https://doi.org/10.3923/rjvs.2008.16.27
Elmahallawy EK, Elshopakey GE, Saleh AA, Agil A, El-Morsey A, Dina MM, El-Shewehy DMM, Ahmed S, Sad AS, Yanai T, Abdo W (2020) S-Methylcysteine (SMC) ameliorates intestinal hepatic and splenic damage induced by cryptosporidium parvuminfection via targeting inflammatory modulators and oxidative stress in Swiss albino mice. Biomedicines 8:423. https://doi.org/10.3390/biomedicines8100423
Lean IS, McDonald V, Pollok RC (2002) The role of cytokines in the pathogenesis of Cryptosporidium infection. Curr Opin Infect Dis 15(3):229–234. https://doi.org/10.1097/00001432-200206000-00003
Lacroix-Lamande S, Mancassola R, Naciri M, Laurent F (2002) Role of gamma interferon in chemokine expression in the ileum of mice and in a murine intestinal epithelial cell line after Cryptosporidium parvum infection. Infect Immun 70:2090–2099
Franchimont D (2004) Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann N Y Acad Sci 1024:124–137. https://doi.org/10.1196/annals.1321.009
Robinson P, Okhuysen PC, Chappell CL, Lewis DE, DE, Shahab I, Andrzej Janecki A, White JR (2001) Expression of tumor necrosis factor α and interleukin 1 β in jejuna of volunteers after experimental challenge with Cryptosporidium parvum correlates with exposure but not with symptoms. Infect Immun 69(2):1172–1174. https://doi.org/10.1128/IAI.69.2.1172-1174
De Sablet T, Potiron L, Marquis M, Bussière FI, Lacroix-Lamandé S, Laurent F (2016) Cryptosporidium parvum increases intestinal permeability through interaction with epithelial cells and IL-1β and TNFα released by inflammatory monocytes. Cell Microbiol 18(12):1871–1880. https://doi.org/10.1111/cmi.12632
Pantsulaia La, Iobadze M, Pantsulaia N, Chikovani T (2014) The effect of citrus peel extracts on cytokines levels and T regulatory cells in acute liver injury. Biomed Res Int 2014:127879. https://doi.org/10.1155/2014/127879
Chaidedgumjorn A, Sotanaphun U, Kitcharoen N, Asavapichayont P, Satiraphan M, Sriamornsak P (2009) Pectins from Citrus maxima. Pharm Biol 47(6):521–526. https://doi.org/10.1155/2014/127879
Chen J, Zhang C, Xia Q, Liu D, Tan X, Li Y, Cao Y (2020) Treatment with subcritical water-hydrolyzed Citrus pectin ameliorated cyclophosphamide-induced immunosuppression and modulated gut microbiota composition in ICR mice. Molecules 25:1302. https://doi.org/10.3390/molecules25061302
Escobedo-Avellaneda Z, Gutiérrez-Uribe J, Valdez-Fragoso A, Torres JA, Welti-Chanes J (2014) Phytochemicals and antioxidant activity of juice, flavedo, albedo and comminuted orange. J Funct Foods 6:470–481. https://doi.org/10.1016/j.jff.2013.11.013
Acknowledgements
The authors wish to acknowledge the assistance given by the National Center of Radiation, Research and Technology, which provided all facilities for this study. Also, the acknowledgment was extended to the contribution of Dr. Sayed Abdel Rahiem, Assistant Professor of Pathology, Faculty of Medicine, Alazhar University, for assistance in setting up histopathological study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not- for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hafez, E.N., Hamed, W.F.A.E. The Efficacy of Citrus maxima Peels Aqueous Extract Against Cryptosporidiosis in Immunecompromised Mice. Acta Parasit. 66, 638–653 (2021). https://doi.org/10.1007/s11686-020-00315-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-020-00315-x