Abstract
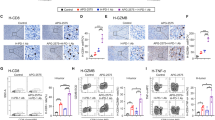
Anti-CD19 chimeric antigen receptor (CAR)-T cell therapy has achieved 40%–50% long-term complete response in relapsed or refractory diffuse large B-cell lymphoma (DLBCL) patients. However, the underlying mechanism of alterations in the tumor microenvironments resulting in CAR-T cell therapy failure needs further investigation. A multi-center phase I/II trial of anti-CD19 CD28z CAR-T (FKC876, ChiCTR1800019661) was conducted. Among 22 evaluable DLBCL patients, seven achieved complete remission, 10 experienced partial remissions, while four had stable disease by day 29. Single-cell RNA sequencing results were obtained from core needle biopsy tumor samples collected from long-term complete remission and early-progressed patients, and compared at different stages of treatment. M2-subtype macrophages were significantly involved in both in vivo and in vitro anti-tumor functions of CAR-T cells, leading to CAR-T cell therapy failure and disease progression in DLBCL. Immunosuppressive tumor microenvironments persisted before CAR-T cell therapy, during both cell expansion and disease progression, which could not be altered by infiltrating CAR-T cells. Aberrant metabolism profile of M2-subtype macrophages and those of dysfunctional T cells also contributed to the immunosuppressive tumor microenvironments. Thus, our findings provided a clinical rationale for targeting tumor microenvironments and reprogramming immune cell metabolism as effective therapeutic strategies to prevent lymphoma relapse in future designs of CAR-T cell therapy.
Similar content being viewed by others
References
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 2018; 378(5): 449–459
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR, Ho PJ, Mielke S, Magenau JM, Holte H, Pantano S, Pacaud LB, Awasthi R, Chu J, Anak Ö, Salles G, Maziarz RT; JULIET Investigators. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019; 380(1): 45–56
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-Cell lymphoma. N Engl J Med 2017; 377(26): 2531–2544
Singh N, Lee YG, Shestova O, Ravikumar P, Hayer KE, Hong SJ, Lu XM, Pajarillo R, Agarwal S, Kuramitsu S, Orlando EJ, Mueller KT, Good CR, Berger SL, Shalem O, Weitzman MD, Frey NV, Maude SL, Grupp SA, June CH, Gill S, Ruella M. Impaired death receptor signaling in leukemia causes antigen-independent resistance by inducing CAR T-cell dysfunction. Cancer Discov 2020; 10(4): 552–567
Orlando EJ, Han X, Tribouley C, Wood PA, Leary RJ, Riester M, Levine JE, Qayed M, Grupp SA, Boyer M, De Moerloose B, Nemecek ER, Bittencourt H, Hiramatsu H, Buechner J, Davies SM, Verneris MR, Nguyen K, Brogdon JL, Bitter H, Morrissey M, Pierog P, Pantano S, Engelman JA, Winckler W. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med 2018; 24(10): 1504–1506
Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, Sussman R, Lanauze C, Ruella M, Gazzara MR, Martinez NM, Harrington CT, Chung EY, Perazzelli J, Hofmann TJ, Maude SL, Raman P, Barrera A, Gill S, Lacey SF, Melenhorst JJ, Allman D, Jacoby E, Fry T, Mackall C, Barash Y, Lynch KW, Maris JM, Grupp SA, Thomas-Tikhonenko A. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov 2015; 5(12): 1282–1295
Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, Steevens NN, Chaney C, Soma L, Chen X, Yeung C, Wood B, Li D, Cao J, Heimfeld S, Jensen MC, Riddell SR, Maloney DG. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016; 126(6): 2123–2138
Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, Boesteanu AC, Wang Y, O’Connor RS, Hwang WT, Pequignot E, Ambrose DE, Zhang C, Wilcox N, Bedoya F, Dorfmeier C, Chen F, Tian L, Parakandi H, Gupta M, Young RM, Johnson FB, Kulikovskaya I, Liu L, Xu J, Kassim SH, Davis MM, Levine BL, Frey NV, Siegel DL, Huang AC, Wherry EJ, Bitter H, Brogdon JL, Porter DL, June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24(5): 563–571
Yan ZX, Li L, Wang W, OuYang BS, Cheng S, Wang L, Wu W, Xu PP, Muftuoglu M, Hao M, Yang S, Zhang MC, Zheng Z, Li J, Zhao WL. Clinical efficacy and tumor microenvironment influence in a dose-escalation study of anti-CD19 chimeric antigen receptor T cells in refractory B-cell non-Hodgkin’s lymphoma. Clin Cancer Res 2019; 25(23): 6995–7003
Zola H, MacArdle PJ, Bradford T, Weedon H, Yasu H, Kurosawa Y. Preparation and characterization of a chimeric CD19 monoclonal antibody. Immunol Cell Biol 1991; 69(6): 411–422
Shen R, Xu PP, Wang N, Yi HM, Dong L, Fu D, Huang JY, Huang HY, Janin A, Cheng S, Wang L, Zhao WL. Influence of oncogenic mutations and tumor microenvironment alterations on extranodal invasion in diffuse large B-cell lymphoma. Clin Transl Med 2020; 10(7): e221
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018; 378(5): 439–448
Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA 2013; 110(50): 20212–20217
Rossi J, Paczkowski P, Shen YW, Morse K, Flynn B, Kaiser A, Ng C, Gallatin K, Cain T, Fan R, Mackay S, Heath JR, Rosenberg SA, Kochenderfer JN, Zhou J, Bot A. Preinfusion polyfunctional anti-CD19 chimeric antigen receptor T cells are associated with clinical outcomes in NHL. Blood 2018; 132(8): 804–814
Lee D, Hokinson D, Park S, Elvira R, Kusuma F, Lee JM, Yun M, Lee SG, Han J. ER stress induces cell cycle arrest at the G2/M phase through eIF2α phosphorylation and GADD45α. Int J Mol Sci 2019; 20(24): 6309
Song G, Xu S, Zhang H, Wang Y, Xiao C, Jiang T, Wu L, Zhang T, Sun X, Zhong L, Zhou C, Wang Z, Peng Z, Chen J, Wang X. TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer Res 2016; 35(1): 148
Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat Rev Cancer 2020; 20(9): 516–531
Hamers AAJ, Pillai AB. A sweet alternative: maintaining M2 macrophage polarization. Sci Immunol 2018; 3(29): eaav7759
Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science 2020; 368(6487): eaaw5473
Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab 2019; 30(1): 36–50
Eil R, Vodnala SK, Clever D, Klebanoff CA, Sukumar M, Pan JH, Palmer DC, Gros A, Yamamoto TN, Patel SJ, Guittard GC, Yu Z, Carbonaro V, Okkenhaug K, Schrump DS, Linehan WM, Roychoudhuri R, Restifo NP. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 2016; 537(7621): 539–543
Xiao Z, Dai Z, Locasale JW. Metabolic landscape of the tumor microenvironment at single cell resolution. Nat Commun 2019; 10(1): 3763
Nakazawa MS, Keith B, Simon MC. Oxygen availability and metabolic adaptations. Nat Rev Cancer 2016; 16(10): 663–673
Chang CH, Curtis JD, Maggi LBJr, Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, Pearce EL. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013; 153(6): 1239–1251
Cham CM, Gajewski TF. Glucose availability regulates IFN-γ production and p70S6 kinase activation in CD8+ effector T cells. J Immunol 2005; 174(8): 4670–4677
Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, Harmrolfs K, Sandouk A, Hesse C, Castro CN, Bähre H, Tschirner SK, Gorinski N, Gohmert M, Mayer CT, Huehn J, Ponimaskin E, Abraham WR, Müller R, Lochner M, Sparwasser T. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat Med 2014; 20(11): 1327–1333
Deng Q, Han G, Puebla-Osorio N, Ma MCJ, Strati P, Chasen B, Dai E, Dang M, Jain N, Yang H, Wang Y, Zhang S, Wang R, Chen R, Showell J, Ghosh S, Patchva S, Zhang Q, Sun R, Hagemeister F, Fayad L, Samaniego F, Lee HC, Nastoupil LJ, Fowler N, Eric Davis R, Westin J, Neelapu SS, Wang L, Green MR. Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat Med 2020; 26(12): 1878–1887
Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, Tonc E, Schreiber RD, Pearce EJ, Pearce EL. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 2015; 162(6): 1229–1241
Acknowledgements
This study was funded in part by research funding from the National Natural Science Foundation of China (Nos. 81830007, 82130004, 81600155, and 81670716), Clinical Research Plan of SHDC (No. 2020CR1032B), Shanghai Rising-Star Program (No. 19QA145600), Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (No. 2017YQ075), Talent (Class A) of Guangci Excellence Youth Plan (No. GCQN-2019-A16), Clinical Research Plan of Shanghai Hospital Development Center (No. SHDC2020CR1032B), Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant Support (Nos. 20152206 and 20152208), China CAR-T Clinical Research Fund Project (No. CARTFR-05), and Samuel Waxman Cancer Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Zixun Yan, Li Li, Di Fu, Wen Wu, Niu Qiao, Yaohui Huang, Lu Jiang, Depei Wu, Yu Hu, Huilai Zhang, Pengpeng Xu, Shu Cheng, Li Wang, Sahin Lacin, Muharrem Muftuoglu, and Weili Zhao have no relevant conflicts of interest to disclose. All procedures followed were in accordance with the ethical standards of Ethics Committee, Ruijin Hospital (approval No. 2018-76) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yan, Z., Li, L., Fu, D. et al. Immunosuppressive tumor microenvironment contributes to tumor progression in diffuse large B-cell lymphoma upon anti-CD19 chimeric antigen receptor T therapy. Front. Med. 17, 699–713 (2023). https://doi.org/10.1007/s11684-022-0972-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-022-0972-8