Abstract
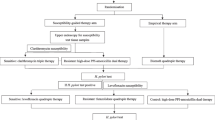
An effective eradication therapy of Helicobacter pylori (H. pylori) should be used for the first time. In this study, we assessed whether tailored therapy based on antibiotic susceptibility testing is more effective than traditional therapy. We also evaluated the factors that cause treatment failure in high-resistance areas. For this multicenter trial, we recruited 467 H. pylori-positive patients. The patients were randomly assigned to receive tailored triple therapy (TATT), tailored bismuth-containing quadruple therapy (TABQT), or traditional bismuth-containing quadruple therapy (TRBQT). For the TATT and TABQT groups, antibiotic selection proceeded via susceptibility testing using an agar-dilution test. The patients in the TRBQT group were given amoxicillin, clarithromycin, esomeprazole, and bismuth. Successful eradication was defined as a negative 13C-urea breath test at least eight weeks after the treatment ended. Susceptibility testing was conducted using an agar-dilution test. The eradication rate was examined via intention-to-treat (ITT) and per-protocol (PP) analyses. The clarithromycin, levofloxacin, and metronidazole resistance rates were 26.12%, 28.69%, and 96.79%, respectively. Resistance against amoxicillin and furazolidone was rare. The eradication rates for TATT, TRBQT, and TABQT were 67.32%, 63.69%, and 85.99% in the ITT analysis (P 0.001) and 74.64%, 68.49%, and 91.22% in the PP analysis (P 0.001), respectively. The efficacy of TABQT was affected by clarithromycin resistance, and bismuth exerted a direct influence on TATT failure. TABQT was the most efficacious regimen for use in high-resistance regions, especially among clarithromycin-susceptible patients.
Similar content being viewed by others
References
Lopes D, Nunes C, Martins MC, Sarmento B, Reis S. Eradication of Helicobacter pylori: past, present and future. J Control Release 2014; 189: 169–186
Graham DY, Fagoonee S, Pellicano R. Increasing role for modified bismuth-containing quadruple therapies for Helicobacter pylori eradication. Minerva Gastroenterol Dietol 2017; 63(2): 77–79
Lee YC, Chen TH, Chiu HM, Shun CT, Chiang H, Liu TY, Wu MS, Lin JT. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut 2013; 62(5): 676–682
Chinese Helicobacter pylori Study Group. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. Chin J Integr Med (Zhonghua Nei Ke Za Zhi) 2012; 10: 832–837 (in Chinese)
Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015; 64: 1353–1367
Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection - the Maastricht V/Florence Consensus Report. Gut 2017; 66(1): 6–30
Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol 2014; 12(2): 177–86.e3, Discussion e12-e13
Sebghatollahi V, Soheilipour M, Khodadoostan M, Shavakhi A, Shavakhi A. Levofloxacin-containing versus clarithromycin-containing therapy for Helicobacter pylori eradication: a prospective randomized controlled clinical trial. Adv Biomed Res 2018; 7(1): 55
Zhang WD, Xiao SD, Hu FL, Hu PJ, Xu ZM. Common agreement on several topics on H. pylori. J New Med (Yi Xue Xin Zhi Za Zhi) 2000; 10(4): 169–170 (in Chinese)
Hu F. Confusion and consensus in the treatment of Helicobacter pylori infection. Chin J Prac Intern Med (Zhongguo Shi Yong Nei Ke Za Zhi) 2005; 25(3): 281–283 (in Chinese)
Hu FL, Hu PJ, Liu WZ, De Wang J, Lv NH, Xiao SD, Zhang WD, Cheng H, Xie Y. Third Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis 2008; 9(3): 178–184
De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Ierardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis 2010; 19(4): 409–414
Yang NM, Meng F, Xu SH, Jiang Y, Li HZ, Zhang XF, Guo F, Wu JS, Li WP, Ji ZZ, Ye LP, Pan J, Chen GL, Ye B, Mao JL, Lin L, Zhang JK, Wang S, Ou YH, Zhu XJ, Lv LH, Yang JH, Shi ZC, Lin CP, Xu F, Wang QY, Mao JB, Li YM. Therapeutic strategies based on clinical big data of antibiotic resistance monitoring of Helicobacter pylori in Zhejiang Province. Chin J Dig Endose (Zhonghua Xiao Hua Nei Jing Za Zhi) 2016; 33(11): 738–742 (in Chinese)
Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol 2008; 5(6): 321–331
Hung IF, Chan P, Leung S, Chan FS, Hsu A, But D, Seto WK, Wong SY, Chan CK, Gu Q, Tong TS, Cheung TK, Chu KM, Wong BC. Clarithromycin-amoxycillin-containing triple therapy: a valid empirical first-line treatment for Helicobacter pylori eradication in Hong Kong? Helicobacter 2009; 14(6): 505–511
Tong YF, Lv J, Ying LY, Xu F, Qin B, Chen MT, Meng F, Tu MY, Yang NM, Li YM, Zhang JZ. Seven-day triple therapy is a better choice for Helicobacter pylori eradication in regions with low antibiotic resistance. World J Gastroenterol 2015; 21(46): 13073–13079
Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon ATR, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ; European Helicobacter Study Group. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut 2012; 61(5): 646–664
Liou JM, Bair MJ, Chen CC, Lee YC, Chen MJ, Chen CC, Tseng CH, Fang YJ, Lee JY, Yang TH, Luo JC, Wu JY, Chang WH, Chang CC, Chen CY, Chen PY, Shun CT, Hsu WF, Hung HW, Lin JT, Chang CY, Wu MS; Taiwan Gastrointestinal Disease and Helicobacter Consortium. Levofloxacin sequential therapy vs. levofloxacin triple therapy in the second-line treatment of Helicobacter pylori: a randomized trial. Am J Gastroenterol 2016; 111(3): 381–387
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing 2009; Nineteenth Informational Supplement M100-S19, A07
Osato MS, Reddy R, Reddy SG, Penland RL, Graham DY. Comparison of the Etest and the NCCLS-approved agar dilution method to detect metronidazole and clarithromycin resistant Helicobacter pylori. Int J Antimicrob Agents 2001; 17(1): 39–44
Lu H, Zhang W, Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: lessons from China. Eur J Gastroenterol Hepatol 2013; 25(10): 1134–1140
Ji Z, Han F, Meng F, Tu M, Yang N, Zhang J. The association of age and antibiotic resistance of Helicobacter pylori: a study in Jiaxing City, Zhejiang Province, China. Medicine (Baltimore) 2016; 95(8): e2831
Liang J, Li J, Han Y, Xia J, Yang Y, Li W, Zhang S, Wu Y, Yuan Y, Li Z, Du Y, Chen M, Chen B, Jiang B, Bai Y, Wen Q, Wu K, Fan D. Helicobacter pylori eradication with ecabet sodium, omeprazole, amoxicillin, and clarithromycin versus bismuth, omeprazole, amoxicillin, and clarithromycin quadruple therapy: a randomized, open-label, phase IV trial. Helicobacter 2012; 17(6): 458–465
Sun Q, Liang X, Zheng Q, Liu W, Xiao S, Gu W, Lu H. High efficacy of 14-day triple therapy-based, bismuth-containing quadruple therapy for initial Helicobacter pylori eradication. Helicobacter 2010; 15(3): 233–238
Qasim A, O’Morain CA. Treatment of Helicobacter pylori infection and factors influencing eradication. Aliment Pharmacol Ther 2002; 16: 24–30
Wermeille J, Cunningham M, Dederding JP, Girard L, Baumann R, Zelger G, Buri P, Metry JM, Sitavanc R, Gallaz L, Merki H, Godin N. Failure of Helicobacter pylori eradication: is poor compliance the main cause? Gastroenterol Clin Biol 2002; 26(3): 216–219
Liang X, Xu X, Zheng Q, Zhang W, Sun Q, Liu W, Xiao S, Lu H. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol 2013; 11(7): 802–807.e1
Xie Y, Zhu Y, Zhou H, Lu ZF, Yang Z, Shu X, Guo XB, Fan HZ, Tang JH, Zeng XP, Wen JB, Li XQ, He XX, Ma JH, Liu DS, Huang CB, Xu NJ, Wang NR, Lu NH. Furazolidone-based triple and quadruple eradication therapy for Helicobacter pylori infection. World J Gastroenterol 2014; 20(32): 11415–11421
Dai C, Li D, Gong L, Xiao X, Tang S. Curcumin ameliorates furazolidone-induced DNA damage and apoptosis in human hepatocyte L02 cells by inhibiting ROS production and mitochondrial pathway. Molecules 2016; 21(8): E1061
Acknowledgements
This study is supported by the Science and Technology Program of Wenzhou (No. 2014S0193), Public Technology Application Research of Zhejiang Province Science and Technology Hall (No. 2014C33246), Medical and Health Plan of Zhejiang Province (No. 2015DTA020), and Public Technology Application Research of Zhejiang Province Science and Technology Hall (No. 2016C33232).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Jie Pan, Zhengchao Shi, Dingsai Lin, Ningmin Yang, Fei Meng, Lang Lin, Zhencheng Jin, Qingjie Zhou, Jiansheng Wu, Jianzhong Zhang, and Youming Li declare that they have no conflict of interest. This trial was approved by the Chinese Ethics Committee of Registering Clinical Trials and the clinical trial registration number was ChiCTR-TRC-13004223 (Date of registration: September 29, 2013) and the approved number of ethic committee was ChiECRCT-2013034 (Date of approved by ethic committee: December 2, 2013). Informed consent was obtained from all patients in this article.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pan, J., Shi, Z., Lin, D. et al. Is tailored therapy based on antibiotic susceptibility effective ? a multicenter, open-label, randomized trial. Front. Med. 14, 43–50 (2020). https://doi.org/10.1007/s11684-019-0706-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-019-0706-8