Abstract
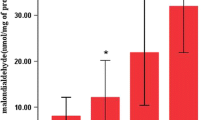
It is well known that high concentration oxygen exposure is a model of acute lung injury (ALI). However, controversy exists over the mechanism. This study was designed to clarify the cellular characteristics in bronchoalveolar lavage fluid (BALF) and body weight loss of rats exposed to oxygen(> 90%). Young male Wistar rats, aged 6 weeks, were divided into three groups: (1) room air group (exposed to room air, n = 22); (2) hyperoxia < 48 h group (exposed to over 90% oxygen for less than 48 h, n = 18); (3) hyperoxia 66–72 h group (exposed to over 90% oxygen for 66–72 h group, n = 7). Compared to the room air group, the total cell counts in the hyperoxia 66–72 h group decreased, whereas the neutrophils increased significantly. The body weights of the rats exposed to room air continued to increase. However, the body weights of oxygen-exposed rats increased slightly on the first day and weight loss was seen from the second day. All rats were noted to have bilateral pleural effusion in the hyperoxia 66–72 h group. The data suggests that (1) an increase in neutrophil count is an evident feature of hyperoxia-induced lung injury; (2) high concentration oxygen exposure can give rise to anorexia and malnutrition, which may play a role in hyperoxia-induced lung injury. Blocking neutrophil influx into lung tissue in the early phase and improving malnutrition are two effective methods to reduce hyperoxic lung injury.
Similar content being viewed by others
References
Valenca S S, Kloss M L, Bezerra F S, Lanzetti M, Silva F L, Porto L C. Effects of hyperoxia on Wistar rat lungs. J Bras Pneumol, 2007, 33(6): 655–662
Desai L P, Sinclair S E, Chapman KE, Hassid A, Waters CM. High tidal volume mechanical ventilation with hyperoxia alters alveolar type II cell adhesion. Am J Physiol Lung Cell Mol Physiol, 2007, 293(3): 769–778
Pagano A, Metrailler R I, Aurrand L M, Lucattelli M, Donati Y, Argiroffo C B. Poly(ADP-ribose) polymerase-1 (PARP-1) controls lung cell proliferation and repair after hyperoxia-induced lung damage. Am J Physiol Lung Cell Mol Physiol, 2007, 293(3): 619–629
Altemeier W A, Sinclair S E. Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care, 2007, 13(1): 73–78
Downey G P, Dong Q, Kruger J, Dedhar S, Cherapanov V. Regulation of neutrophil activation in acute lung injury. Chest, 1999, 116(1 Suppl): 46S–54S
Auten R L, Whorton M H, Mason S N. Blocking neutrophil influx reduces DNA damage in hyperoxia-exposed newborn rat lung. Am J Respir Cell Mol Boil, 2002, 26(4): 391–397
Perkowski S, Scherpereel A, Murciano J C, Arguiri E, Solomides C C, Albelda S M, Muzykantov V, Christofidou S M. Dissociation between alveolar transmigration of neutrophils and lung injury in hyperoxia. Am J Physiol Lung Cell Mol Physiol, 2006, 291(5): 1050–1058
Mackarel A J, Cottell D C, Russell K J, Gerald M X, Connor C M. Migration of neutrophils across human pulmonary endothelial cells is not blocked by matrix metalloproteinase or serine protease inhibitors. Am J Respir Cell Mol Biol, 1999, 20(6): 1209–1219
Lee W L, Downey G P. Leukocyte elastase: physiological functions and role in acute lung injury. Am J Respir Crit Care Med, 2001, 164(5): 896–904
Hirche T O, Atkinson J J, Bahr S, Belaaouaj A. Deficiency in neutrophil elastase does not impair neutrophil recruitment to inflamed sites. Am J Respir Cell Mol Biol, 2004, 30(4): 576–584
Delacourt C, Herigault S, Delclaux C, Poncin A, Levame M, Harf A, Saudubray F, Lafuma C. Protection against acute lung injury by intravenous or intratracheal pretreatment with EPI-HNE-4, a new potent neutrophil elastase inhibitor. Am J Respir Cell Mol Biol, 2002, 26(3): 290–297
Yamada M, Kubo H, Kobayashi S, Ishizawa K, Sasaki H. Interferon-γ: a key contributor to hyperoxia-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol, 2004, 287(5): L1042–L1047
Deng H, Mason SN, Auten R L. Lung inflammation in hyperoxia can be prevented by anti-chemokine treatment in newborn rats. Am J Respir Crit Care Med, 2000, 162(6): 2316–2323
Vozzelli M A, Mason S N, Whorton M H, Auten R L. Antimacrophage chemokine treatment prevents neutrophil and macrophage influx in hyperoxia-exposed newborn rat lung. Am J Physiol Lung Cell Mol Physiol, 2004, 286(3): L488–493
Peterson M W, Walter M E, Nygaard S D. Effect of neutrophil mediators on epithelial permeability. Am J Respir Cell Mol Biol, 1995, 13(6): 719–727
Fischer B M, Voynow J A. Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am J Respir Cell Mol Biol, 2002, 26(4): 447–452
Aoshiba K, Yasuda K, Yasui S, Tamaoki J, Nagai A. Serine proteases increase oxidative stress in lung cells. Am J Physiol Lung Cell Mol Physiol, 2001, 281(3): L556–564
Bhandari V, Choo W R, Homer R J, Elias J A. Increased hyperoxia-induced mortality and acute lung injury in IL-13 null mice. J Immunol, 2007, 178(8): 4993–5000
Bellmeyer A, Martino J M, Chandel N S, Scott G R, Dean D A, Mutlu G M. Leptin resistance protects mice from hyperoxia-induced acute lung injury. Am J Respir Crit Care Med, 2007, 175(6): 587–594
Barazzone-Argiroffo C, Muzzin P, Donati Y R, Kan C D, Aubert M L, Piguet P F. Hyperoxia increases leptin production: a mechanism mediated through endogenous elevation of corticosterone. Am J Physiol Lung Cell Mol Physiol, 2001, 281(5): L1150–L1156
Factor P, Ridge K, Alverdy J, Jacob I. Continuous enteral nutrition attenuates pulmonary edema in rats exposed to 100% oxygen. J Appl Physiol, 2000, 89(5): 1759–1765
Yamaoka S, Kim H S, Ogihara T, Oue S, Takitani K, Yoshida Y, Tamai H. Severe vitamin E deficiency exacerbates acute hyperoxic lung injury associated with increased oxidative stress and inflammation. Free Radic Res, 2007, 42(6): 602–612
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, X., Zhao, W. Cellular changes in bronchoalveolar lavage fluid in hyperoxia-induced lung injury. Front. Med. China 2, 370–373 (2008). https://doi.org/10.1007/s11684-008-0071-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-008-0071-5