Abstract
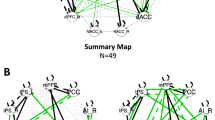
Tobacco cigarette smoking is associated with disrupted brain network dynamics in resting brain networks including the Salience (SN) and Fronto parietal (FPN). Unified multimodal methods [Resting state connectivity analysis, Diffusion Tensor Imaging (DTI), neurite orientation dispersion and density imaging (NODDI), and cortical thickness analysis] were employed to test the hypothesis that the impact of cigarette smoking on the balance among these networks is due to alterations in white matter connectivity, microstructural architecture, functional connectivity and cortical thickness (CT) and that these metrics define fundamental differences between people who smoke and nonsmokers. Multimodal analyses of previously collected 7 Tesla MRI data via the Human Connectome Project were performed on 22 people who smoke (average number of daily cigarettes was 10 ± 5) and 22 age- and sex-matched nonsmoking controls. First, functional connectivity analysis was used to examine SN-FPN-DMN interactions between people who smoke and nonsmokers. The anatomy of these networks was then assessed using DTI and CT analyses while microstructural architecture of WM was analyzed using the NODDI toolbox. Seed-based connectivity analysis revealed significantly enhanced within network [p = 0.001 FDR corrected] and between network functional coupling of the salience and R-frontoparietal networks in people who smoke [p = 0.004 FDR corrected]. The network connectivity was lateralized to the right hemisphere. Whole brain diffusion analysis revealed no significant differences between people who smoke and nonsmokers in Fractional Anisotropy, Mean diffusivity and in neurite orienting and density. There were also no significant differences in CT in the hubs of these networks. Our results demonstrate that tobacco cigarette smoking is associated with enhanced functional connectivity, but anatomy is largely intact in young adults. Whether this enhanced connectivity is pre-existing, transient or permanent is not known. The observed enhanced connectivity in resting state networks may contribute to the maintenance of smoking frequency.



Similar content being viewed by others
Data availability
Not applicable.
References
Baeza-Loya, S., Velasquez, K.M., Molfese, D.L., Viswanath, H., Curtis, K.N., Thompson-Lake, D.G., Baldwin, P.R., Ellmore, T.M., De La Garza, R. 2nd, & Salas, R. (2016) Anterior cingulum white matter is altered in tobacco smokers. The American Journal on Addictions, 25(3), 210-4.
Basser, P. J., & Pierpaoli, C. (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance Series B, 111(3), 209–219.
Behzadi, Y., Restom, K., Liaum, J., & Liu, T.T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37(1), 90–101.
Breckel, T.P., Thiel, C.M., & Giessing, C. (2013) The efficiency of functional brain networks does not differ between smokers and non-smokers. Psychiatry Research, 214(3), 349-56.
Bucholz, K. K., Cadoret, R., Cloninger, C. R., et al. (1994). A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. Journal of Studies on Alcohol., 55(2), 149–158.
Chand, G.B., & Dhamala, M. (2017). Interactions between the anterior cingulate-insula network and the fronto-parietal network during perceptual decision-making. Neuroimage, 152, 381-389.
Chen, Z., Guo, Y., Suo, T., & Feng, T. (2018). Coupling and segregation of large-scale brain networks predict individual differences in delay discounting. Biological Psychology, 133, 63–71.
Clewett, D., Luo, S., Hsu, E., Ainslie, G., Mather, M., & Monterosso, J. (2014). Increased functional coupling between the left fronto-parietal network and anterior insula predicts steeper delay discounting in smokers. Human Brain Mapping, 35(8), 3774–3787.
Cooper, N., Garcia, J. O., Tompson, S. H., O’Donnell, M. B., Falk, E. B., & Vettel, J. M. (2018). Time-evolving dynamics in brain networks forecast responses to health messaging. Network Neuroscience, 3(1), 138–156.
Fagerström, K. (2012). Determinants of tobacco use and renaming the FTND to the fagerström test for cigarette dependence. Nicotine & Tobacco Research, 14, 75–78. https://doi.org/10.1093/ntr/ntr137
Glasser, M.F., Sotiropoulos, S.N., Wilson, J.A., Coalson, T.S., Fischl, B., Andersson, J.L., Xu, J., Jbabdi, S., Webster, M., Polimeni, J.R., Van Essen, D.C., Jenkinson, M., WU-Minn HCP Consortium (2013). The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage, 80, 105–24. https://doi.org/10.1016/j.neuroimage.2013.04.127
Gogliettino, A. R., Potenza, M. N., & Yip, S. W. (2016). White matter development and tobacco smoking in young adults: A systematic review with recommendations for future research. Drug and Alcohol Dependence, 1(162), 26–33.
Hesselbrock, M., Easton, C., Bucholz, K. K., Schuckit, M., & Hesselbrock, V. (1999). A validity study of the SSAGA-a comparison with the SCAN. Addiction, 94(9), 1361–1370.
Hudkins, M., O'Neill, J., Tobias, M.C., Bartzokis, G., London, E.D. (2012). Cigarette smoking and white matter microstructure. Psychopharmacology (Berl), 221(2), 285-95.
Janes, A. C., de Frederick, B., Richardt, S., Burbridge, C., Merlo-Pich, E., Renshaw, P. F., Evins, A. E., Fava, M., & Kaufman, M. J. (2009). Brain fMRI reactivity to smoking-related images before and during extended smoking abstinence. Experimental and Clinical Psychopharmacology, 17(6), 365–373.
Janes, A. C., Nickerson, L. D., & Frederick Bde, B. (2012). Kaufman MJ Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug and Alcohol Dependence, 125(3), 252–259.
Janes, A. C., Farmer, S., Peechatka, A. L., Frederick Bde, B., & Lukas, S. E. (2015). Insula-dorsal anterior cingulate cortex coupling is associated with enhanced brain reactivity to smoking cues. Neuropsychopharmacology, 40(7), 1561–1568.
Keeley, R.J., Prillaman, M.E., Scarlata, M., Vrana, A., Tsai, P.J., Gomez, J.L., Bonaventura, J., Lu, H., Michaelides, M., & Stein, E.A. (2022). Adolescent nicotine administration increases nicotinic acetylcholine receptor binding and functional connectivity in specific cortico-striatal-thalamic circuits. Brain Communications, 4(6):fcac291.
Kenny, P. J., & Markou, A. (2006). Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology, 31(6), 1203–1211.
Kenny, P. J., Chartoff, E., Roberto, M., Carlezon, W. A., Jr., & Markou, A. (2009). NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: Role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology, 34(2), 266–281.
Li, Y., Yuan, K., Cai, C., Feng, D., Yin, J., Bi, Y., Shi, S., Yu, D., Jin, C., von Deneen, K. M., Qin, W., & Tian, J. (2015). Reduced frontal cortical thickness and increased caudate volume within fronto-striatal circuits in young adult smokers. Drug and Alcohol Dependence, 1(151), 211–219.
Menon, V. (2011). Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506.
Menon, V., & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5–6), 655–667.
Morales, A. M., Ghahremani, D., Kohno, M., Hellemann, G. S., & London, E. D. (2014). Cigarette exposure, dependence, and craving are related to insula thickness in young adult smokers. Neuropsychopharmacology, 39(8), 1816–22.
Rae, C. L., Davies, G., Garfinkel, S. N., Gabel, M. C., Dowell, N. G., Cercignani, M., Seth, A. K., Greenwood, K. E., Medford, N., & Critchley, H. D. (2017). Deficits in neurite density underlie white matter structure abnormalities in first-episode psychosis. Biological Psychiatry, 82(10), 716–725.
Smallwood, J., Bernhardt, B. C., Leech, R., Bzdok, D., Jefferies, E., & Margulies, D. S. (2021). The default mode network in cognition: A topographical perspective. Nature Reviews Neuroscience, 22(8), 503–513.
Stoeckel, L. E., Chai, X. J., Zhang, J., & Whitfield-Gabrieli, S. (2016). Evins AE Lower gray matter density and functional connectivity in the anterior insula in smokers compared with never smokers. Addiction Biology, 21(4), 972–981.
Stolerman, I. P., Mirza, N. R., & Shoaib, M. (1995). Nicotine psychopharmacology: Addiction, cognition and neuroadaptation. Medicinal Research Reviews, 15(1), 47–72.
Van Essen, D.C., Smith, S.M., Barch, D.M., Behrens, T.E., Yacoub, E., Ugurbil, K.; WU-Minn HCP Consortium. (2013). The WU-Minn Human Connectome Project: an overview. Neuroimage, 80, 62–79.
Vu, A. T., Auerbach, E., Lenglet, C., Moeller, S., Sotiropoulos, S. N., Jbabdi, S., Andersson, J., Yacoub, E., & Ugurbil, K. (2015). High resolution whole brain diffusion imaging at 7T for the Human Connectome Project. NeuroImage, 15(122), 318–331.
Whitfield-Gabrieli, S., Moran, J. M., Nieto-Castanon, A., Triantafyllou, C., Saxe, R., & Gabrieli, J. D. (2011). Associations and dissociations between default and self-reference networks in the human brain. NeuroImage, 55, 225–232.
Whitfield-Gabrieli, S., & Nieto-Castanon, A. (2012). CONN: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125-41.
Yang, H., Long, X. Y., Yang, Y., Yan, H., Zhu, C. Z., Zhou, X. P., Zang, Y. F., & Gong, Q. Y. (2007). Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. NeuroImage, 36, 144–152.
Yu, D., Yuan, K., Zhang, B., Liu, J., Dong, M., Jin, C., Luo, L., Zhai, J., Zhao, L., Zhao, Y., Gu, Y., Xue, T., Liu, X., Lu, X., & Qin, W. (2016). Tian J White matter integrity in young smokers: A tract-based spatial statistics study. Addiction Biology, 21(3), 679–687.
Zhang, T., Zhang, L., Liang, Y., Siapas, A. G., Zhou, F. M., & Dani, J. A. (2009). Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. Journal of Neuroscience, 29(13), 4035–4043.
Funding
I wish to acknowledge the financial support of the Louis V Gerstner foundation that supported this study. Funding was also provided by the Rising star grant at the University of Texas.
Author information
Authors and Affiliations
Contributions
Dr. Francis did the Image Analysis, Statistical analysis and wrote the first draft. Dr. Sebille did the computational programming, Image analysis and helped with editing the manuscript. Dr. Gabrieli did the quality control of the neuroimaging data and helped with manuscript edits. Dr. Camprodon's helped with data interpretation and with the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Internal Review Boards at Washington university at Saint Louis University of Minnesota.
Human ethics and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Francis, A.N., Sebille, S., Whitfield-Gabrieli, S. et al. Multimodal 7T imaging reveals enhanced functional coupling between salience and frontoparietal networks in young adult tobacco cigarette smokers. Brain Imaging and Behavior (2024). https://doi.org/10.1007/s11682-024-00882-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s11682-024-00882-x