Abstract
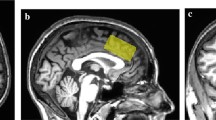
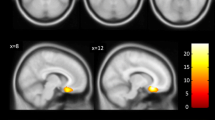
Brain-derived neurotrophic factor (BDNF) is the most abundant brain neurotrophin and plays a critical role in neuronal growth, survival and plasticity, implicated in the pathophysiology of Bipolar Disorders (BD). The single-nucleotide polymorphism in the BDNF gene (BDNF rs6265) has been associated with decreased hippocampal BDNF secretion and volume in met carriers in different populations, although the val allele has been reported to be more frequent in BD patients. The anterior cingulate cortex (ACC) is a key center integrating cognitive and affective neuronal connections, where consistent alterations in brain metabolites such as Glx (Glutamate + Glutamine) and N-acetylaspartate (NAA) have been consistently reported in BD. However, little is known about the influence of BDNF rs6265 on neurochemical profile in the ACC of Healthy Controls (HC) and BD subjects. The aim of this study was to assess the influence of BDNF rs6265 on ACC neurometabolites (Glx, NAA and total creatine- Cr) in 124 euthymic BD type I patients and 76 HC, who were genotyped for BDNF rs6265 and underwent a 3-Tesla proton magnetic resonance imaging and spectroscopy scan (1 H-MRS) using a PRESS ACC single-voxel (8cm3) sequence. BDNF rs6265 polymorphism showed a significant two-way interaction (diagnosis × genotype) in relation to NAA/Cr and total Cr. While met carriers presented increased NAA/Cr in HC, BD-I subjects with the val allele revealed higher total Cr, denoting an enhanced ACC metabolism likely associated with increased glutamatergic metabolites observed in BD-I val carriers. However, these results were replicated only in men. Therefore, our results support evidences that the BDNF rs6265 polymorphism exerts a complex pleiotropic effect on ACC metabolites influenced by the diagnosis and sex.




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author on reasonable request.
References
American Psychiatric Association, Ed, T., & Revision (2000). Diagnostic and statistical Manual of Mental Disorders (Fourth.). Washington, DC: American Psychiatric Publishing, Inc.
Baj, G., Carlino, D., Gardossi, L., & Tongiorgi, E. (2013). Toward a unified biological hypothesis for the BDNF Val66Met-associated memory deficits in humans: a model of impaired dendritic mRNA trafficking. Frontiers in Neuroscience, 7, 188.
Boulle, F., et al. (2012). Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Molecular Psychiatry, 17(6), 584–596.
Brown, N. C., Andreazza, A. C., & Young, L. T. (2014). An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Research, 218, 61–68.
Buonocore, M. H., & Maddock, R. J. (2015). Magnetic resonance spectroscopy of the brain: a review of physical principles and technical methods. Reviews in the neurosciences, 26(6), 609–632.
Camuso, S., La Rosa, P., Fiorenza, M. T., & Canterini, S. (2022). Pleiotropic effects of BDNF on the cerebellum and hippocampus: implications for neurodevelopmental disorders. Neurobiology of Disease, 163, 105606.
Clay, H. B., Sillivan, S., & Konradi, C. (2011). Mitochondrial dysfunction and Pathology in Bipolar Disorder and Schizophrenia. International Journal of Developmental Neuroscience, 29, 311–324.
Chen, Z-Y; Patel, P.D; Sant, G; Meng, C-X; Teng, K.K; Hempstead, B.L; Lee, F.S. (2004). Variant Brain-Derived Neurotrophic Factor (BDNF) (Met66) Alters the Intracellular Trafficking and Activity-Dependent Secretion of Wild-Type BDNF in Neurosecretory Cells and Cortical Neurons. The Journal of Neuroscience, 24(18):4401–4411.
Croarkin, P. E., Thomas, M. A., Port, J. D., Baruth, J. M., Choi, D. S., Abulseoud, O. A., & Frye, M. A. (2015). N-acetylaspartate normalization in bipolar depression after lamotrigine treatment. Bipolar Disorders, 17, 450–457.
Decoster, J., van Os, J., Kenis, G., Henquet, C., Peuskens, J., De Hert, M., & van Winkel, R. (2011). Age at Onset of psychotic disorder: Cannabis, BDNF Val66Met and sex-specific models of gene–environment Interaction. American Journal of Medical Genetics, 156, 363–369.
De-Paula, V. J., Gattaz, W. F., & Forlenza, O. V. (2016). Long-term lithium treatment increases intracellular and extracellular brain-derived neurotrophic factor (BDNF) in cortical and hippocampal neurons at subtherapeutic concentrations. Bipolar Disorders, 00, 1–4. https://doi.org/10.1111/bdi.12449
Di Rosa, M. C., Zimbone, S., Saab, M. W., & Tomasello, M. F. (2021). The pleiotropic potential of BDNF beyond neurons: implication for a healthy mind in a healthy body. Life, 11, 1256.
Egan, M. F., Kojima, M., Callicott, J. H., Goldberg, T. E., Kolachana, B. S., Bertolino, A., Zaitsev, E., Gold, B., Goldman, D., Dean, M., Lu, B., & Weinberger, D. R. (2003). The BDNF val66met polymorphism affects activitydependent secretion of BDNF and human memory and hippocampal function. Cell, 112, 257–269.
Ehrlich, A., Schubert, F., Pehrs, C., & Gallinat, J. (2015). Alterations of cerebral glutamate in the euthymic state of patients with bipolar disorder. Psychiatry Research, 233(2), 73–80.
Frey, B. N., Walss-Bass, C., Stanley, J. A., Nery, F. G., Matsuo, K., Nicoletti, M. A., Hatch, J. P., Bowden, C. L., Escamilla, M. A., & Soares, J. C. (2007). Brain-derived neurotrophic factor val66met polymorphism a¡ects prefrontal energy metabolism in bipolar disorder. Neuroreport, 18, 1567–1570.
First, M. B., Spitzer, R. L., & Williams, J. B. (1996). Structured clinical interview for DSM-IV Axis I Disorders SCID-I. Washington, DC: American Psychiatric Press.
Fukumoto, N., Fujii, T., Combarros, O., Kamboh, M. I., Tsai, S. J., Matsushita, S., Nacmias, B., Comings, D. E., Arboleda, H., Ingelsson, M., Hyman, B. T., Akatsu, H., Grupe, A., Nishimura, A., Zatz, M., Mattila, K. M., Rinne, J., Goto, Y. I., Asada, T., Nakamura, S., & Kunugi, H. (2010). Sexually dimorphic effect of the Val66Met polymorphism of BDNF on susceptibility to Alzheimer’s disease: new data and meta-analysis. American Journal of Medical Genetics, 153B, 235–242.
Gallinat, G., Schubert, F., Brühl, R., Hellweg, R., Klär, A. A., Kehrer, C., Wirth, C., Sander, T., & Lang, U. E. (2010). Met carriers of BDNF Val66Met genotype show increased N-acetylaspartate concentration in the anterior cingulate cortex. Neuroimage, 49, 767–771.
Gasparovic, C., Song, T., Devier, D., Bockholt, H. J., Caprihan, A., Mullins, P. G., et al. (2006). Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnetic Resonance In Medicine, 55(6), 1219–1226. https://doi.org/10.1002/mrm.20901
Gruber, O., Hasan, A., Scherk, H., Wobrock, T., Schneider-Axmann, T., Ekawardhani, S., Schmitt, A., Backens, M., Reith, W., Meyer, J., & Falkai, P. (2012). Association of the brain-derived neurotrophic factor val66met polymorphism with magnetic resonance spectroscopic markers in the human hippocampus: in vivo evidence for effects on the glutamate system. European Archives of Psychiatry and Clinical Neuroscience, 262, 23–31.
Hamilton, M. (1967). Development of a rating scale for primary depressive illness. The British Journal Of Social And Clinical Psychology, 6, 278–296.
Harrisberger, F., Spalek, K., Smieskova, R., Schmidt, A., Coynel, D., Milnik, A., Fastenrath, M., Freytag, V., Gschwindc, L., Walter, A., Vogel, T., Bendfeldt, K., de Quervaina, D. J. F., Papassotiropoulos, A., & Borgwardt, S. (2014). The association of the BDNF Val66Met polymorphism and the hippocampal volumes in healthy humans: a joint meta-analysis of published and new data. Neuroscience and Biobehavioral Reviews, 42, 267–278.
Harrisberger, F., Smieskova, R., Schmidt, A., Lenz, C., Walter, A., Wittfeld, K., Grabe, H. J., Lang, U. E., Fusar-Poli, P., & Borgwardt, S. (2015). BDNF Val66Met polymorphism and hippocampal volume in neuropsychiatric disorders: a systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews, 55, 107–118.
Harrison, P. J., Hall, N., Mould, A., Al-Juffali, N., & Tunbridge, E. M. (2021). Cellular calcium in bipolar disorder: systematic review and meta-analysis. Molecular Psychiatry, 26, 4106–4116.
Hibar, D. P., Westlye, L. T., Doan, N. T., Jahanshad, N., Cheung, J. W., Ching, C. R. K., et al. (2017). Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA bipolar disorder Working Group. Molecular Psychiatry, 00, 1–11.
Hofer, M., Pagliusi, S. R., Hohn, A., Leibrock, J., & Barde, Y. A. (1990). Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO Journal, 9, 2459–2464.
Hosang, G. M., Shiles, C., Tansey, K. E., Mcguffin, P., and Uher, R. (2014). Interaction between stress and the BDNF Val66Met polymorphism in depression: a systematic review and meta-analysis. BMC Med. 12:7. https://doi.org/10.1186/1741-7015-12-7
Karaca, M., Frigerio, F., Migrenne, S., Martin-Levilain, J., Skytt, D. M., Pajecka, K., Martin-del-Rio, R., Gruetter, R., Tamarit-Rodriguez, J., Waagepetersen, H. S., Magnan, C., & Maechler, P. (2015). GDH-Dependent glutamate oxidation in the brain dictates peripheral energy substrate distribution. Cell Reports, 13, 365–375.
Kennedy, K. G., Shahatit, Z., Dimick, M. K., Fiksenbaum, L., Freeman, N., Zai, C. C., Kennedy, J. L., MacIntosh, B. J., & Goldstein, B. I. (2021). Neurostructural correlates of BDNF rs6265 genotype in youth bipolar disorder. Bipolar Disorders, 00, 1–10.
Kowiaoski, P., Lietzau, G., Czuba, E., Waśkow, M., Steliga, A., & Moryś, J. (2017). BDNF: a key factor with multipotent impact on Brain Signaling and synaptic plasticity. Cellular and Molecular Neurobiology, 1, 15.
Kreis, R. (2004). Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. Nmr In Biomedicine, 17(6), 361–381. https://doi.org/10.1002/nbm.891
Laing, K. R., Mitchell, D., Wersching, H., Czira, M. E., Berger, K., & Baune, B. T. (2012). Brain-derived neurotrophic factor (BDNF) gene: a gender-specific role in cognitive function during normal cognitive aging of the MEMO-Study? Age, 34, 1011–1022.
Laitinen, J., Samarut, J., Hölttä, E. (1994) A nontoxic and versatile protein salting-out method for isolation of DNA. Biotechniques 17(2), 316–322.
Lang, U. E., Hellweg, R., Sander, T., & Gallinat, J. (2009). The Met allele of the BDNF Val66Met polymorphism is associated with increased BDNF serum concentrations. Molecular Psychiatry, 14, 120–122.
Li, M., Chang, H., & Xiao, X. (2016). BDNF Val66Met polymorphism and bipolar disorder in european populations: a risk association in case-control, family based and GWAS studies. Neuroscience & Biobehavioral Reviews, 68, 218–233.
Maletic, V., & Raison, C. (2014). Integrated Neurobiology of Bipolar Disorder. Frontiers in Psychiatry, 5, 98.
Mandolini, G. M., Lazzaretti, M., Pigoni, A., Delvecchio, G., Soares, J. C., & Brambilla, P. (2019). The impact of BDNF Val66Met polymorphism on cognition in bipolar disorder: a review. Journal of Affective Disorders, 243, 552–558.
Markham, A., Bains, R., Franklin, P., & Spedding, M. (2014). Changes in mitochondrial function are pivotal in neurodegenerative and psychiatric disorders: how important is BDNF? British Journal of Pharmacology, 171, 2206–2229.
Martens, L., Herrmann, L., Colic, L., Li, M., Richter, A., Behnisch, G., Stork, O., Seidenbecher, C., Schott, B. H., & Walter, M. (2021). Met carriers of the BDNF Val66Met polymorphism show reduced Glx/ NAA in the pregenual ACC in two independent cohorts. Scientifc Reports, 11, 6742.
Mlynárik, V., Gruber, S., & Moser, E. (2001). Proton T (1) and T (2) relaxation times of human brain metabolites at 3 Tesla. Nmr In Biomedicine, 14(5), 325–331. https://doi.org/10.1002/nbm.713
Mitchelmore, C., & Gede, L. (2014). Brain derived neurotrophic factor: epigenetic regulation in psychiatric disorders. Brain Research, 1586, 162–172.
Neves-Pereira, M., Mundo, E., Muglia, P., King, N., Macciardi, F., & Kennedy, J. L. (2002). The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. American Journal of Human Genetics, 71, 651–655.
Nortje, G., Stein, D.J., Radua, J., Mataix-Cols, D., Horn, N., (2013). Systematic review and voxel-based metaanalysis of diffusion tensor imaging studies in bipolar disorder. J Affec Disor, 150, 192–200.
Pagani, R., Gasparini, A., Ielmini, M., Caselli, M., Poloni, I., Ferrari, N., Marino, M., & Callegari, F., C (2019). Twenty years of Lithium pharmacogenetics: a systematic review. Psychiatry Research, 278, 42–50.
Paul, P., Nadella, R. K., Sen, S., Ithal, D., Mahadevan, J., Reddy, Y. C. J., Jain, S., Purushottam, M., & Viswanath, B. (2021). Association study of BDNF Val66Met gene polymorphism with bipolar disorder and lithium treatment response in indian population. Journal of Psychopharmacology, 35(12), 1510–1516.
Pedersen, C.B., Mors, O., Bertelsen, A., Waltoft, BL; Agerbo, E; McGrath, J.J; Mortensen,P.B;; Eaton, W.W. A Comprehensive Nationwide Study of the Incidence Rate and Lifetime Risk for Treated Mental Disorders. JAMA Psychiatry, 71(5), 573–581.
Pereira, L. P., Köhler, C. A., de Sousa, R. T., Solmi, M., de Freitas, B. P., Fornaro, M., Machado-Vieira, R., Miskowiak, K. W., Vieta, E., Veronese, N., Stubbs, B., & Carvalho, A. F. (2017). The relationship between genetic risk variants with brain structure and function in bipolar disorder: a systematic review of genetic neuroimaging studies. Neuroscience & Biobehavioral Reviews, 79, 87–109.
Provencher, S.W.S., (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med, 30(6), 672–679.
Pruunsild, P., Kazantseva, A., Aid, T., Palm, K., & Timmusk, T. (2007). Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics, 90, 397–406.
Rackayova, V., Cudalbu, C., Pouwels, P. J. W., & Braissant, O. (2017). Creatine in the central nervous system: from magnetic resonance spectroscopy to creatine deficiencies. Analytical Biochemistry, 529, 144e157.
Smedler, E., Pålsson, E., Hashimoto, K., & Landén, M. (2021). Association of CACNA1C polymorphisms with serum BDNF levels in bipolar disorder. The British Journal of Psychiatry, 218, 77–79. https://doi.org/10.1192/bjp.2019.173
Scotti-Muzzi, E., Umla-Runge, K., & Soeiro-de-Souza, M. G. (2021). Anterior cingulate cortex neurometabolites in bipolar disorder are influenced by mood state and medication: a meta-analysis of 1H-MRS studies. European Neuropsychopharmacology, 47, 62–73.
Scotti-Muzzi, E., Chile, T., Vallada, H., Otaduy, M. C. G., & Soeiro-de-Souza, M. G. (2022). Association between CACNA1C gene rs100737 polymorphism and glutamatergic neurometabolites in bipolar disorder. European Neuropsychopharmacology, 59, 26–35.
Sheehan, D.V., Lecrubier, Y., Sheehan, K.H., Amorim, P., Janavs, J., Weiller, E. et al. (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a struc tured diagnostic psychiatric interview for DSMIV and ICD-10. J Clin Psychiatry, 59(Suppl 20), 22–33.
Sklar, P., Gabriel, S. B., Mcinnis, M. G., Bennett, P., Lim, Y., Tsan, G., et al. (2002). Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Molecular Psychiatry, 7, 579–593.
Soeiro-de-Souza, M. G., Andreazza, A. C., Carvalho, A. F., Machado-Vieira, R., Young, T., & Moreno, R. A. (2013). Number of manic episodes is associated with elevated DNA oxidation in bipolar I disorder. International Journal of Neuropsychopharmacology, 16, 1505–1512.
Soeiro-de-Souza, M. G., Otaduy, M. C. G., Machado-Vieira, R., Moreno, R. A., Nery, F. G., Leite, C., & Lafer, B. (2018a). Anterior cingulate cortex glutamatergic metabolites and Mood Stabilizers in Euthymic Bipolar I disorder patients: a Proton magnetic resonance spectroscopy study. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(12), 985–991.
Soeiro-de-Souza, M. G., Otaduy, M. C. G., Machado-Vieira, R., Moreno, R. A., Nery, F. G., Leite, C., & Lafer, B. (2018b). Lithium-associated anterior cingulate neurometabolic profile in euthymic bipolar I disorder: a 1 H-MRS study. Journal of Affective Disorders, 241, 192–199.
Stanisz, G. J., Odrobina, E. E., Pun, J., Escaravage, M., Graham, S. J., Bronskill, M. J., et al. (2005). T1, T2 relaxation and magnetization transfer in tissue at 3T. Magnetic Resonance In Medicine, 54(3), 507–512. https://doi.org/10.1002/mrm.20605
Stern, A. J., Savostyanova, A. A., Goldman, A., Barnett, A. S., van der Veen, J. W. C., Callicott, J. H., Mattay, V. S., Weinberger, D. R., & Marenco, S. (2008). Impact of the brain derived neurotrophic factor Val66Met polymorphism on levels of hippocampal N-Acetyl aspartate assessed by magnetic resonance spectroscopic imaging at 3 Tesla. Biological Psychiatry, 15(10), 856–862.
Stork, C., & Renshaw, P. F. (2005). Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Molecular Psychiatry, 10, 900–919.
Strakowski, S. M., Adler, C. M., Almeida, J., Altshuler, L. L., Blumberg, H. P., Chang, K. D., et al. (2012). The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disorders, 14, 313–325.
Tsai, S. J. (2018). Critical issues in BDNF Val66Met genetic studies of Neuropsychiatric Disorders. Frontiers in Molecular Neuroscience, 11, 56.
Vederine, F., Wessa, M., Leboyer, M., Houenou, J., (2011). A meta?analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog. Neuropsychopharmacol Biol. Psychiatry, 35, 1820–1826.
Walls, A. B., Waagepetersen, H. S., Bak, L. K., Schousboe, A., & Sonnewald, U. (2015). The glutamine–glutamate/GABA cycle: function, Regional differences in glutamate and GABA production 72 European Neuropsychopharmacology 47 (2021) 62–73 and effects of interference with GABA metabolism. Neurochem. Res. 40(2), 402–409.
Wei, S. W., & Berman, K. F. (2019). Ovarian hormones, genes, and the brain: the case of estradiol and the brain-derived neurotrophic factor (BDNF) gene. Neuropsychopharmacology : Official Publication Of The American College Of Neuropsychopharmacology, 44, 223–224.
Young, R. C., Biggs, J. T., Ziegler, V. E., & Meyer, D. A. (1978). A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry, 133, 429–435.
Yu, A. C., Schousboe, A., & Hertz, L. (1982). Metabolic fate of 14 C-labeled glutamate in astrocytes in primary cultures. Journal Of Neurochemistry, 39, 954–960.
Acknowledgements
This study was supported by the São Paulo Research Foundation. We thank the University of São Paulo for all its support and the team of researchers, patients and volunteers that participated in this long-term study. The authors report no biomedical financial interests or potential conflicts of interest.
Funding
The Sao Paulo Research Foundation (FAPESP) financed this study (2012/23796-2 and 2010/12286-8).
Author information
Authors and Affiliations
Contributions
Conception and study design (ES-M, MCGO, MGS-S), data collection or acquisition (E-SM, TC, HV, MCGO, MGS-S), statistical analysis (ES-M and MGS-S), interpretation of results (ES-M and MGS-S), drafting the manuscript or revising it critically for important intellectual content (ES-M, MCGO, MGS-S), and approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the study (E-SM, TC, HV, MCGO, MGS-S).
Corresponding author
Ethics declarations
Ethical approval
The Research Ethics Committee of the University of São Paulo approved the study.
Consent to participate
Written informed consent was obtained from all study participants.
Consent to publish
All those listed as authors are qualified for authorship and all who are qualified to be authors are listed as authors on the byline.
Competing interests
None of the authors report biomedical financial interests or potential conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scotti-Muzzi, E., Chile, T., Vallada, H. et al. BDNF rs6265 differentially influences neurometabolites in the anterior cingulate of healthy and bipolar disorder subjects. Brain Imaging and Behavior 17, 282–293 (2023). https://doi.org/10.1007/s11682-023-00757-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-023-00757-7