Abstract
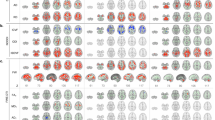
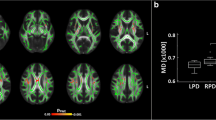
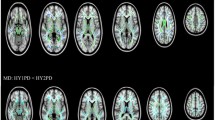
Myelinated white matter showing diamagnetic susceptibility is important for information transfer in the brain. In Parkinson’s disease (PD), the white matter is also suffering degenerative alterations. Quantitative susceptibility mapping (QSM) is a novel technique for noninvasive assessment of regional white matter ultrastructure, and provides different information of white matter in addition to standard diffusion tensor imaging (DTI). In this study, we used QSM to detect spatial white matter alterations in PD patients (n = 65) and age- and sex-matched normal controls (n = 46). Voxel-wise tract-based spatial statistics were performed to analyze QSM and DTI data. QSM showed extensive white matter involvement—including regions adjacent to the frontal, parietal, and temporal lobes—in PD patients, which was more widespread than that observed using DTI. Both QSM and DTI showed similar alterations in the left inferior longitudinal fasciculus and right cerebellar hemisphere. Further, alterations in the white matter were correlated with motor impairment and global disease severity in PD patients. We suggest that QSM may provide a novel approach for detecting white matter alterations and underlying network disruptions in PD. Further, the combination of QSM and DTI would provide a more complete evaluation of the diseased brain by analyzing different biological tissue properties.






Similar content being viewed by others
References
Aarsland, D., Andersen, K., Larsen, J. P., Lolk, A., Nielsen, H., & Kragh-Sorensen, P. (2001). Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology, 56, 730–736.
Agosta, F., Canu, E., Stefanova, E., Sarro, L., Tomic, A., Spica, V., Comi, G., Kostic, V. S., & Filippi, M. (2014). Mild cognitive impairment in Parkinson’s disease is associated with a distributed pattern of brain white matter damage. Human Brain Mapping, 35, 1921–1929.
Agosta, F., Canu, E., Stojkovic, T., Pievani, M., Tomic, A., Sarro, L., Dragasevic, N., Copetti, M., Comi, G., Kostic, V. S., & Filippi, M. (2013). The topography of brain damage at different stages of Parkinson’s disease. Human Brain Mapping, 34, 2798–2807.
Argyridis, I., Li, W., Johnson, G. A., & Liu, C. (2013). Quantitative magnetic susceptibility of the developing mouse brain reveals microstructural changes in the white matter. Neuroimage, 88C, 134–142.
Auning, E., Kjaervik, V. K., Selnes, P., Aarsland, D., Haram, A., Bjornerud, A., Hessen, E., Esnaashari, A., & Fladby, T. (2014). White matter integrity and cognition in Parkinson’s disease: a cross-sectional study. British Medical Journal Open, 4, e3976.
Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomedicine, 15, 435–455.
Braak, H., Del, T. K., Rub, U., de Vos, R. A., Jansen, S. E., & Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging, 24, 197–211.
Braak, H., Ghebremedhin, E., Rub, U., Bratzke, H., & Del, T. K. (2004). Stages in the development of Parkinson’s disease-related pathology. Cell and Tissue Research, 318, 121–134.
Chen, B., Fan, G. G., Liu, H., & Wang, S. (2015). Changes in anatomical and functional connectivity of Parkinson’s disease patients according to cognitive status. European Journal of Radiology, 84, 1318–1324.
Delano-Wood, L., Stricker, N. H., Sorg, S. F., Nation, D. A., Jak, A. J., Woods, S. P., Libon, D. J., Delis, D. C., Frank, L. R., & Bondi, M. W. (2012). Posterior cingulum white matter disruption and its associations with verbal memory and stroke risk in mild cognitive impairment. Journal of Alzheimers Disease, 29, 589–603.
Du, G., Liu, T., Lewis, M. M., Kong, L., Wang, Y., Connor, J., Mailman, R. B., & Huang, X. (2015) Quantitative susceptibility mapping of the midbrain in Parkinson’s disease. Movement Disorders.
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., & Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR American Journal of Roentgenology, 149, 351–356.
Gallagher, C., Bell, B., Bendlin, B., Palotti, M., Okonkwo, O., Sodhi, A., Wong, R., Buyan-Dent, L., Johnson, S., Willette, A., Harding, S., Ninman, N., Kastman, E., & Alexander, A. (2013). White matter microstructural integrity and executive function in Parkinson’s disease. Journal of the International Neuropsychological Society, 19, 349–354.
Gattellaro, G., Minati, L., Grisoli, M., Mariani, C., Carella, F., Osio, M., Ciceri, E., Albanese, A., & Bruzzone, M. G. (2009). White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. AJNR American Journal of Neuroradiology, 30, 1222–1226.
Guan, X., Xuan, M., Gu, Q., Huang, P., Liu, C., Wang, N., Xu, X., Luo, W., & Zhang, M. (2017a) Regionally progressive accumulation of iron in Parkinson’s disease as measured by quantitative susceptibility mapping. NMR in Biomedicine, 30(4). https://doi.org/10.1002/nbm.3489.
Guan, X., Xuan, M., Gu, Q., Xu, X., Huang, P., Wang, N., Shen, Z., Xu, J., Luo, W., & Zhang, M. (2017b). Influence of regional iron on the motor impairments of Parkinson’s disease: A quantitative susceptibility mapping study. Journal of Magnetic Resonance Imaging, 45, 1335–1342.
Harrison, M. B., Wylie, S. A., Frysinger, R. C., Patrie, J. T., Huss, D. S., Currie, L. J., & Wooten, G. F. (2009). UPDRS activity of daily living score as a marker of Parkinson’s disease progression. Movement Disorders, 24, 224–230.
He, N., Ling, H., Ding, B., Huang, J., Zhang, Y., Zhang, Z., Liu, C., Chen, K., & Yan, F. (2015). Region-specific disturbed iron distribution in early idiopathic Parkinson’s disease measured by quantitative susceptibility mapping. Human Brain Mapping, 36, 4407–4420.
Hobson, P., & Meara, J. (2004). Risk and incidence of dementia in a cohort of older subjects with Parkinson’s disease in the United Kingdom. Movement Disorders, 19, 1043–1049.
Hughes, A. J., Daniel, S. E., Kilford, L., & Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Journal of Neurology Neurosurgery, and Psychiatry, 55, 181–184.
Jeurissen, B., Leemans, A., Tournier, J. D., Jones, D. K., & Sijbers, J. (2013). Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Human Brain Mapping, 34, 2747–2766.
Jones, D. K., Knosche, T. R., & Turner, R. (2013). White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage, 73, 239–254.
Kamagata, K., Motoi, Y., Tomiyama, H., Abe, O., Ito, K., Shimoji, K., Suzuki, M., Hori, M., Nakanishi, A., Sano, T., Kuwatsuru, R., Sasai, K., Aoki, S., & Hattori, N. (2013). Relationship between cognitive impairment and white-matter alteration in Parkinson’s disease with dementia: tract-based spatial statistics and tract-specific analysis. European Radiology, 23, 1946–1955.
Karagulle, K. A., Lehericy, S., Luciana, M., Ugurbil, K., & Tuite, P. (2008). Altered diffusion in the frontal lobe in Parkinson disease. AJNR American Journal of Neuroradiology, 29, 501–505.
Katzman, R., Zhang, M. Y., Ouang-Ya Qu, Wang, Z. Y., Liu, W. T., Yu, E., Wong, S. C., Salmon, D. P., & Grant, I. (1988). A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. Journal of Clinical Epidemiology, 41, 971–978.
Langley, J., Huddleston, D. E., Merritt, M., Chen, X., McMurray, R., Silver, M., Factor, S. A., & Hu, X. (2016). Diffusion tensor imaging of the substantia nigra in Parkinson’s disease revisited. Human Brain Mapping, 37, 2547–2556.
Lee, J., Shmueli, K., Fukunaga, M., van Gelderen, P., Merkle, H., Silva, A. C., & Duyn, J. H. (2010) Sensitivity of MRI resonance frequency to the orientation of brain tissue microstructure. Proceeding of the National Academy of Science of the United States of America 107: 5130–5135.
Li, W., & Liu, C. (2013). Comparison of Magnetic Susceptibility Tensor and Diffusion Tensor of the Brain. Journal of Neuroscience and Neuroengineer, 2, 431–440.
Li, W., Wang, N., Yu, F., Han, H., Cao, W., Romero, R., Tantiwongkosi, B., Duong, T. Q., & Liu, C. (2015). A method for estimating and removing streaking artifacts in quantitative susceptibility mapping. Neuroimage, 108, 111–122.
Li, W., Wu, B., Avram, A. V., & Liu, C. (2012). Magnetic susceptibility anisotropy of human brain in vivo and its molecular underpinnings. Neuroimage, 59, 2088–2097.
Li, W., Wu, B., Batrachenko, A., Bancroft-Wu, V., Morey, R. A., Shashi, V., Langkammer, C., De Bellis, M. D., Ropele, S., Song, A. W., & Liu, C. (2014). Differential developmental trajectories of magnetic susceptibility in human brain gray and white matter over the lifespan. Human Brain Mapping, 35, 2698–2713.
Li, W., Wu, B., & Liu, C. (2011). Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. Neuroimage, 55, 1645–1656.
Li, X., Vikram, D. S., Lim, I. A., Jones, C. K., Farrell, J. A., & van Zijl, P. C. (2012). Mapping magnetic susceptibility anisotropies of white matter in vivo in the human brain at 7 T. Neuroimage, 62, 314–330.
Liu, C. (2010). Susceptibility tensor imaging. Magn Reson Med, 63, 1471–1477.
Liu, T., Spincemaille, P., de Rochefort, L., Kressler, B., & Wang, Y. (2009). Calculation of susceptibility through multiple orientation sampling (COSMOS): a method for conditioning the inverse problem from measured magnetic field map to susceptibility source image in MRI. Magnetic Resonance in Medicine, 61(1), 196–204.
Liu, C., Li, W., Johnson, G. A., & Wu, B. (2011). High-field (9.4 T) MRI of brain dysmyelination by quantitative mapping of magnetic susceptibility. Neuroimage, 56, 930–938.
Liu, C., Li, W., Wu, B., Jiang, Y., & Johnson, G. A. (2012). 3D fiber tractography with susceptibility tensor imaging. Neuroimage, 59, 1290–1298.
Liu, C., Li, W., Tong, K. A., Yeom, K. W., & Kuzminski, S. (2015). Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. Journal of Magnetic Resonance Imaging, 42, 23–41.
Lodygensky, G. A., Marques, J. P., Maddage, R., Perroud, E., Sizonenko, S. V., Huppi, P. S., & Gruetter, R. (2012). In vivo assessment of myelination by phase imaging at high magnetic field. Neuroimage, 59, 1979–1987.
Martin, W. R., Wieler, M., & Gee, M. (2008). Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology, 70, 1411–1417.
Murakami, Y., Kakeda, S., Watanabe, K., Ueda, I., Ogasawara, A., Moriya, J., Ide, S., Futatsuya, K., Sato, T., Okada, K., Uozumi, T., Tsuji, S., Liu, T., Wang, Y., & Korogi, Y. (2015) Usefulness of Quantitative Susceptibility Mapping for the Diagnosis of Parkinson Disease. In, Vol. 36, pp. 1102–1108.
Neil, J., Miller, J., Mukherjee, P., & Huppi, P. S. (2002). Diffusion tensor imaging of normal and injured developing human brain - a technical review. NMR Biomedicine, 15, 543–552.
Parashos, S. A., Luo, S., Biglan, K. M., Bodis-Wollner, I., He, B., Liang, G. S., Ross, G. W., Tilley, B. C., & Shulman, L. M. (2014). Measuring disease progression in early Parkinson disease: the National Institutes of Health Exploratory Trials in Parkinson Disease (NET-PD) experience. JAMA Neurology, 71, 710–716.
Rae, C. L., Correia, M. M., Altena, E., Hughes, L. E., Barker, R. A., & Rowe, J. B. (2012). White matter pathology in Parkinson’s disease: the effect of imaging protocol differences and relevance to executive function. Neuroimage, 62, 1675–1684.
Rudko, D. A., Solovey, I., Gati, J. S., Kremenchutzky, M., & Menon, R. S. (2014). Multiple sclerosis: improved identification of disease-relevant changes in gray and white matter by using susceptibility-based MR imaging. Radiology, 272, 851–864.
Sen, S., Kawaguchi, A., Truong, Y., Lewis, M. M., & Huang, X. (2010). Dynamic changes in cerebello-thalamo-cortical motor circuitry during progression of Parkinson’s disease. Neuroscience, 166, 712–719.
Song, S. K., Sun, S. W., Ramsbottom, M. J., Chang, C., Russell, J., & Cross, A. H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage, 17, 1429–1436.
Surdhar, I., Gee, M., Bouchard, T., Coupland, N., Malykhin, N., & Camicioli, R. (2012). Intact limbic-prefrontal connections and reduced amygdala volumes in Parkinson’s disease with mild depressive symptoms. Parkinsonism & Related Disorders, 18, 809–813.
Tuch, D. S., Reese, T. G., Wiegell, M. R., Makris, N., Belliveau, J. W., & Wedeen, V. J. (2002). High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magnetic Resonance in Medicine, 48, 577–582.
Williams-Gray, C. H., Evans, J. R., Goris, A., Foltynie, T., Ban, M., Robbins, T. W., Brayne, C., Kolachana, B. S., Weinberger, D. R., Sawcer, S. J., & Barker, R. A. (2009). The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain, 132, 2958–2969.
Worker, A., Blain, C., Jarosz, J., Chaudhuri, K. R., Barker, G. J., Williams, S. C., Brown, R. G., Leigh, P. N., Dell’Acqua, F., & Simmons, A. (2014). Diffusion tensor imaging of Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy: a tract-based spatial statistics study. PLoS One, 9, e112638.
Wu, B., Li, W., Guidon, A., & Liu, C. (2012). Whole brain susceptibility mapping using compressed sensing. Magnetic Resonance in Medicine, 67, 137–147.
Yu, H., Sternad, D., Corcos, D. M., & Vaillancourt, D. E. (2007). Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage, 35, 222–233.
Zhan, W., Kang, G. A., Glass, G. A., Zhang, Y., Shirley, C., Millin, R., Possin, K. L., Nezamzadeh, M., Weiner, M. W., Marks, W. J., & Schuff, N. (2012). Regional alterations of brain microstructure in Parkinson’s disease using diffusion tensor imaging. Movement Disorders, 27, 90–97.
Zhang, M. Y., Katzman, R., Salmon, D., Jin, H., Cai, G. J., Wang, Z. Y., Qu, G. Y., Grant, I., Yu, E., Levy, P., & Et, A. (1990). The prevalence of dementia and Alzheimer’s disease in Shanghai, China: impact of age, gender, and education. Annals of Neurology, 27, 428–437.
Acknowledgements
We wish to thank all the participants including patients with Parkinson’s disease and normal volunteers. We also thank the assistance from department of Neurology in our institute. Finally, Xiaojun Guan would like to personally thank Dr. Jingrui Jin, for her infinite patience, care and love.
Funding
This work was supported by the 13th Five-year Plan for National Key Research and Development Program of China (Grant No. 2016YFC1306600), the Fundamental Research Funds for the Central Universities of China (2017XZZX001-01), the 12th Five-year Plan for National Science and Technology Supporting Program of China (Grant No. 2012BAI10B04) and the National Natural Science Foundation of China (Grant Nos. 81571654, 81371519, 81701647 and 81771820). P.H. was supported in part by the Projects of Medical and Health Technology Development Program in Zhejiang Province (2015KYB174) and C.L. was supported in part by the National Institutes of Health through grants NIMH R01MH096979, and by the National Natural Science Foundation of China (Grant No. 81428013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaation and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guan, X., Huang, P., Zeng, Q. et al. Quantitative susceptibility mapping as a biomarker for evaluating white matter alterations in Parkinson’s disease. Brain Imaging and Behavior 13, 220–231 (2019). https://doi.org/10.1007/s11682-018-9842-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-018-9842-z