Abstract
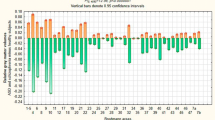
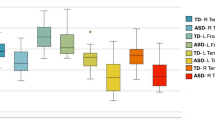
Both autism spectrum disorder (ASD) and schizophrenia are often characterized as disorders of white matter integrity. Multimodal investigations have reported elevated metabolic rates, cerebral perfusion and basal activity in various white matter regions in schizophrenia, but none of these functions has previously been studied in ASD. We used 18fluorodeoxyglucose positron emission tomography to compare white matter metabolic rates in subjects with ASD (n = 25) to those with schizophrenia (n = 41) and healthy controls (n = 55) across a wide range of stereotaxically placed regions-of-interest. Both subjects with ASD and schizophrenia showed increased metabolic rates across the white matter regions assessed, including internal capsule, corpus callosum, and white matter in the frontal and temporal lobes. These increases were more pronounced, more widespread and more asymmetrical in subjects with ASD than in those with schizophrenia. The highest metabolic increases in both disorders were seen in the prefrontal white matter and anterior limb of the internal capsule. Compared to normal controls, differences in gray matter metabolism were less prominent and differences in adjacent white matter metabolism were more prominent in subjects with ASD than in those with schizophrenia. Autism spectrum disorder and schizophrenia are associated with heightened metabolic activity throughout the white matter. Unlike in the gray matter, the vector of white matter metabolic abnormalities appears to be similar in ASD and schizophrenia, may reflect inefficient functional connectivity with compensatory hypermetabolism, and may be a common feature of neurodevelopmental disorders.






Similar content being viewed by others
References
Altamura, A. C., Bertoldo, A., Marotta, G., Paoli, R. A., Caletti, E., Dragogna, F., Buoli, M., Baglivo, V., Mauri, M. C., & Brambilla, P. (2013). White matter metabolism differentiates schizophrenia and bipolar disorder: a preliminary PET study. Psychiatry Research, 214(3), 410–414.
Ameis, S. H., & Catani, M. (2015). Altered white matter connectivity as a neural substrate for social impairment in autism spectrum disorder. Cortex, 62, 158–181.
Amiaz, R., Vainiger, D., Gershon, A. A., Weiser, M., Lavidor, M., & Javitt, D. C. (2016). Applying transcranial magnetic stimulation (TMS) over the dorsal visual pathway induces schizophrenia-like disruption of perceptual closure. Brain Topography, 29(4), 552–560.
Atkinson, J., & Braddick, O. (2011). From genes to brain development to phenotypic behavior: “dorsal-stream vulnerability” in relation to spatial cognition, attention, and planning of actions in Williams syndrome (WS) and other developmental disorders. Progress in Brain Research, 189, 261–283.
Barnea-Goraly, N., Kwon, H., Menon, V., Eliez, S., Lotspeich, L., & Reiss, A. L. (2004). White matter structure in autism: evidence from diffusion tensor imaging. Biological Psychiatry, 55, 323–326.
Bemporad, J. R. (1991). Dementia praecox as a failure of neoteny. Theoretical Medicine and Bioethics, 12(1), 45–51.
Benaglia, T., Chauveau, D., Hunter, D. R., & Young, D. S. (2009). Mixtools: An R package for analyzing finite mixture models. Journal of Statistical Software, 32, 1–29.
Benítez-Burraco, A., & Lattanzi, W. (2017). Schizophrenia and human self-domestication: an evolutionary linguistic approach. Brain, Behavior and Evolution, 89, 162–184.
Benítez-Burraco, A., Lattanzi, W., & Murphy, E. (2016). Language impairments in ASD resulting from failed domestication of the human brain. Frontiers in Neuroscience, 10, 373.
Bennett, M. R., & Lagopoulos, J. (2015). Neurodevelopmental sequelae associated with gray and white matter changes and their cellular basis: a comparison between autism spectrum disorder, ADHD and dyslexia. International Journal of Developmental Neuroscience, 46, 132–143.
Binczyk, F., Stjelties, B., Weber, C., Goetz, M., Meier-Hein, K., Meinzer, H.-P., Bobek-Billewicz, B., Tarnawski, R., & Polanska, J. (2017). MimSeg – an algorithm for automated detection of tumor tissue on NMR apparent diffusion coefficient maps. Information Sciences, 384, 235–248.
Braddick, O., & Atkinson, J. (2013). Visual control of manual actions: brain mechanisms in typical development and developmental disorders. Developmental Medicine & Child Neurology, 55(suppl 4), 13–18.
Braddick, O., Atkinson, J., & Wattam-Bell, J. (2003). Normal and anomalous development of visual motion processing: motion coherence and ‘dorsal stream vulnerability’. Neuropsychologia, 41, 1769–1784.
Bralet, M.-C., Buchsbaum, M. S., DeCastro, A., Hazlett, E. A., Haznedar, M. M., Shihabuddin, L., & Mitelman, S. A. (2016). FDG-PET scans in patients with Kraepelinian and non-Kraepelinian schizophrenia. European Archives of Psychiatry and Clinical Neurosciences, 266(6), 481–494.
Buchsbaum, B. R., Olsen, R. K., Koch, P., & Berman, K. F. (2005). Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processing during verbal working memory. Neuron, 48(4), 687–697.
Buchsbaum, M. S., Buchsbaum, B. R., Hazlett, E. A., Haznedar, M. M., Newmark, R. E., Tang, C. Y., & Hof, P. R. (2007). Relative glucose metabolic rate higher in white matter in patients with schizophrenia. The American Journal of Psychiatry, 164, 1072–1081.
Buchsbaum, M. S., Tang, C. Y., Peled, S., Gudbjartsson, H., Lu, D., Hazlett, E. A., Downhill, J., Haznedar, M. M., Fallon, J. H., & Atlas, S. W. (1998). MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport, 9(3), 425–430.
Buck, D., Förschler, A., Lapa, C., Schuster, T., Vollmar, P., Korn, T., Nessler, S., Stadelmann, C., Drzezga, A., Buck, A. K., Wester, H. J., Zimmer, C., Krause, B. J., & Hemmer, B. (2012). 18F-FDG PET detects inflammatory infiltrates in spinal cord experimental autoimmune encephalomyelitis lesions. Journal of Nuclear Medicine, 53, 1269–1276.
Bullmore, E. T., & Sporns, O. (2012). The economy of brain network organization. Nature Reviews Neuroscience, 13, 336–349.
Butler, P. D., Martinez, A., Foxe, J. J., Kim, D., Zemon, V., Silipo, G., Mahoney, J., Shpaner, M., Jalbrzikowski, M., & Javitt, D. C. (2007). Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain, 130(2), 417–430.
Cheng, H., Newman, S. D., Kent, J. S., Bolbecker, A., Klaunig, M. J., O’Donnell, B. F., Puce, A., & Hetrick, W. P. (2015). White matter abnormalities of microstructure and physiological noise in schizophrenia. Brain Imaging and Behavior, 9(4), 868–877.
Courchesne, E., Press, G. A., & Yeung-Courchesne, R. (1993). Parietal lobe abnormalities detected with MR in patients with infantile autism. American Journal of Roentgenology, 160, 387–393.
Creasey, H., Rumsey, J. M., Schwartz, M., Duara, R., Rapoport, J. L., & Rapoport, S. I. (1986). Brain morphometry in autistic men as measured by volumetric computed tomography. Archives of Neurology, 43, 669–672.
Crespi, B. J. (2013). Developmental heterochrony and the evolution of autistic perception, cognition and behavior. BioMed Central Medicine, 11, 119.
Crespi, B. J., & Leach, E. (2016). The evolutionary biology of human neurodevelopment: evo-neuro-devo comes of age. In J. C. Boughner & C. Rolian (Eds.), Developmental approaches to human evolution (Chap. 5). Wiley-Blackwell, pp. 205–230.
Crow, T. (1995). A Darwinian approach to the origins of psychosis. British Journal of Psychiatry, 167, 12–25.
de Paula Faria, D., de Vries, E. F., Sijbesma, J. W., Buchpiguel, C. A., Dierckx, R. A., & Copray, S. C. (2014). PET imaging of glucose metabolism, neuroinflammation and demyelination in the lysolecithin rat model of multiple sclerosis. Multiple Sclerosis, 20(11), 1443–1452.
Esik, O., Emri, M., Csornai, M., Kasler, M., Godeny, M., & Tron, L. (1999). Radiation myelopathy with partial functional recovery: PET evidence of long-term increased metabolic activity of the spinal cord. Journal of Neurological Sciences, 163, 39–43.
Esik, O., Emri, M., Szakall, S. Jr., Herzog, H., Safrany, G., Lengyel, E., Boer, A., Liszkay, G., Tron, L., Lengyel, Z., & Repa, I. (2004). PET identifies transitional metabolic change in the spinal cord following a subthreshold dose of irradiation. Pathology and Oncology Research, 10, 42–46.
Fitzsimmons, J., Kubicki, M., & Shenton, M. E. (2013). Review of functional and anatomical brain connectivity findings in schizophrenia. Current Opinion in Psychiatry, 26(2), 172–187.
Floeth, F. W., Galldiks, N., Eicker, S., Stoffels, G., Herdmann, J., Steiger, H. J., Antoch, G., Rhee, S., & Langen, K. J. (2013). Hypermetabolism in 18F-FDG PET predicts favorable outcome following decompressive surgery in patients with degenerative cervical myelopathy. Journal of Nuclear Medicine, 54(9), 1577–1583.
Greimel, E., Bartling, J., Dunkel, J., Brüchl, M., Deimel, W., Remschmidt, H., Kamp-Becker, I., & Schulte-Körne, G. (2013). The temporal dynamics of coherent motion processing in autism spectrum disorder: evidence for a deficit in the dorsal pathway. Behavioral Brain Research, 251, 168–175.
Grinter, E. J., Maybery, M. T., & Badcock, D. R. (2010). Vision in developmental disorders: is there a dorsal stream deficit? Brain Research Bulletin, 82(3–4), 147–160.
Hazlett, E. A., Buchsbaum, M. S., Hsieh, P., Haznedar, M. M., Platholi, J., LiCalzi, E. M., Cartwright, C., & Hollander, E. (2004). Regional glucose metabolism within cortical Brodmann areas in healthy individuals and autistic patients. Neuropsychobiology, 49(3), 115–125.
Hazlett, E. A., Byne, W., Brickman, A. M., Mitsis, E. M., Newmark, R., Haznedar, M. M., Knatz, D. T., Chen, A. D., & Buchsbaum, M. S. (2010). Effects of sex and normal aging on regional brain activation during verbal memory performance. Neurobiology of Aging, 31(5), 826–838.
Haznedar, M. M., Buchsbaum, M. S., Hazlett, E. A., LiCalzi, E. M., Cartwright, C., & Hollander, E. (2006). Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. American Journal of Psychiatry, 163(7), 1252–1263.
Hickok, G., & Poeppel, D. (2004). Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition, 92, 67–99.
Jenkinson, M., Bannister, P., Brady, M., & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17, 825–841.
Jones, D. K., Knösche, T. R., & Turner, R. (2013). White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. NeuroImage, 73, 239–254.
Just, M. A., Keller, T. A., Malave, V. L., Kana, R. K., & Varma, S. (2012). Autism as a neural systems disorder: a theory of fronto-posterior underconnectivity. Neuroscience and Biobehavioral Reviews, 36(4), 1292–1313.
Kanaan, R. A., Picchioni, M. M., McDonald, C., Shergill, S. S., & McGuire, P. K. (2017). White matter deficits in schizophrenia are global and don’t progress with age. Australian & New Zealand Journal of Psychiatry, 51(10), 1020–1031.
Karlsgodt, K. H. (2016). Diffusion imaging of white matter in schizophrenia: progress and future directions. Biological Psychiatry, 1(3), 209–217.
Keefe, R. S., Mohs, R. C., Losonszy, M. F., Davidson, M., Silverman, J. M., Kendler, K. S., Horvath, T. B., Nora, R., & Davis, K. L. (1987). Characteristics of very poor outcome schizophrenia. American Journal of Psychiatry, 144, 889–895.
Kelleher, R. J. 3rd, & Bear, M. F. (2008). The autistic neuron: troubled translation? Cell, 135(3), 401–406.
Kenk, M., Selvanathan, T., Rao, N., Suridjan, I., Rusjan, P., Remington, G., Meyer, J. H., Wilson, A. A., Houle, S., & Mizrahi, R. (2015). Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophrenia Bulletin, 41(1), 85–93.
Klyachko, V. A., & Stevens, C. F. (2003). Connectivity optimization and the positioning of cortical areas. Proceedings of the National Academy of Sciences of the United States of America, 100(13), 7937–7941.
Lajiness-O’Neill, R. R., Beaulilieu, I., Titus, J. B., Asamoah, A., Bigler, E. D., Bawle, E. V., & Pollack, R. (2005). Memory and learning in children with 22q11.2 deletion syndrome: evidence for ventral and dorsal stream disruption? Child Neuropsychology, 11(1), 55–71.
Largen, J. W. Jr., Smith, R. C., Calderon, M., Baumgartner, R., Lu, R. B., Schoolar, J. C., & Ravichandran, G. K. (1984). Abnormalities of brain structure and density in schizophrenia. Biological Psychiatry, 19(7), 991–1013.
Laycock, R., Cross, A. J., Dalle Nogare, F., & Crewther, S. G. (2014). Self-rated social skills predict visual perception: impairments in object discrimination requiring transient attention associated with high autistic tendency. Autism Research, 7(1), 104–111.
Lehmann, M., Melbourne, A., Dickson, J. C., Ahmed, R. M., Modat, M., Cardoso, M. J., Thomas, D. L., De Vita, E., Crutch, S. J., Warren, J. D., Mahoney, C. J., Bomanji, J., Hutton, B. F., Fox, N. C., Golay, X., Ourselin, S., & Schott, J. M. (2016). A novel use of arterial spin labelling MRI to demonstrate focal hypoperfusion in individuals with posterior cortical atrophy a multimodal imaging study. Journal of Neurology, Neurosurgery, and Psychiatry, 87(9), 1032–1034.
Lo, Y. C., Chou, T. L., Fan, L. Y., Gau, S. S., Chiu, Y. N., & Tseng, W. Y. (2013). Altered structure-function relations of semantic processing in youths with high-functioning autism: a combined diffusion and functional MRI study. Autism Research, 6(6), 561–570.
London, K., & Howman-Giles, R. (2014). Normal cerebral FDG uptake during childhood. European Journal of Nuclear Medicine and Molecular Imaging, 41(4), 723–735.
Milne, E., Swettenham, J., & Campbell, R. (2005). Motion perception and autistic spectrum disorder: a review. Current Psychology of Cognition, 23, 3–33.
Mitelman, S. A., Bralet, M.-C., Haznedar, M. M., Hollander, E., Shihabuddin, L., Hazlett, E. A., & Buchsbaum, M. S. (2016). Diametrical relationship between gray and white matter volumes in autism spectrum disorder and schizophrenia. Brain Imaging and Behavior. https://doi.org/10.1007/s11682-016-9648-9.
Mitelman, S. A., Bralet, M.-C., Haznedar, M. M., Hollander, E., Shihabuddin, L., Hazlett, E. A., & Buchsbaum, M. S. (2017). Positron emission tomography assessment of cerebral glucose metabolic rates in autism spectrum disorder and schizophrenia. Brain Imaging and Behavior. https://doi.org/10.1007/s11682-017-9721-z.
Mitelman, S. A., Torosjan, Y., Newmark, R. E., Schneiderman, J. S., Chu, K.-W., Brickman, A. M., Haznedar, M. M., Hazlett, E. A., Tang, C. Y., Shihabuddin, L., & Buchsbaum, M. S. (2007). Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophrenia Research, 92, 211–224.
Mori, S., Wakana, S., van Zij, P. C. M., & Nagae-Poetscher, L. M. (2005). MRI atlas of human white matter. Amsterdam: Elsevier.
O’Donoghue, S., Holleran, L., Cannon, D. M., & McDonald, C. (2017). Anatomical dysconnectivity in bipolar disorder compared with schizophrenia: a selective review of structural network analyses using diffusion MRI. Journal of Affective Disorders, 209, 217–228.
Pinkham, A., Loughead, J., Ruparel, K., Wu, W.-C., Overton, E., Gur, R. C., & Gur, R. E. (2011). Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Research, 194(1), 64–72.
Plomp, G., Roinishvili, M., Chkonia, E., Kapanadze, G., Kereselidze, M., Brand, A., & Herzog, M. H. (2013). Electrophysiological evidence for ventral stream deficits in schizophrenia patients. Schizophrenia Bulletin, 39(3), 547–554.
Radu, C. G., Shu, C. J., Shelly, S. M., Phelps, M. E., & Witte, O. N. (2007). Positron emission tomography with computed tomography imaging of neuroinflammation in experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America, 104(6), 1937–1942.
Ramage, A. E., Fox, P. T., Brey, R. L., Narayana, S., Cykowski, M. D., Naqibuddin, M., Sampedro, M., Holliday, S. L., Franklin, C., Wallace, D. J., Weisman, M. H., & Petri, M. (2011). Neuroimaging evidence of white matter inflammation in newly diagnosed systemic lupus erythematosus. Arthritis and Rheumatism, 63(10), 3048–3057.
Reed, L. J., Lasserson, D., Marsden, P., Stanhope, N., Stevens, T., Bello, F., Kingsley, D., Colchester, A., & Kopelman, M. D. (2003). FDG-PET findings in the Wernicke-Korsakoff syndrome. Cortex, 39, 1027–1045.
Roh, J. K., Nam, H., & Lee, M. C. (1998). A case of central pontine and extrapontine myelinolysis: a case report. Case Reports in Neurology, 4, 167–172.
Rønne, F., Tfelt-Hansen, O. C., & Rødam, L. (2017). Central pontine myelinolysis and localized fluorodeoxyglucose uptake seen on 18FDG-PET/CT. World Journal of Nuclear Medicine, 16(1), 56–58.
Rubinov, M., & Bullmore, E. T. (2013). Fledging pathoconnectomics of psychiatric disorders. Trends in Cognitive Sciences, 17(12), 641–647.
Saur, D., Kreher, B. W., Schnell, S., Kümmerer, P., Vry, M.-S., Umarova, R., Musso, M., Glauche, V., Abel, S., Huber, W., Rijntjes, M., Hennig, J., & Weiller, C. (2008). Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the United States of America, 105(46), 18035–18040.
Schiepers, C., Van Hecke, P., Vandenberghe, R., Van Oostende, D., Dupont, P., Demaerel, P., Bormans, G., & Carton, H. (1997). Positron emission tomography, magnetic resonance imaging and proton NMR spectroscopy of white matter in multiple sclerosis. Multiple Sclerosis, 3(1), 8–17.
Sehatpour, P., Dias, E. C., Butler, P. D., Revheim, N., Guilfoyle, D. N., Foxe, J. J., & Javitt, D. C. (2010). Impaired visual object processing across an occipital-frontal hippocampal brain network in schizophrenia: an integrated neuroimaging study. Archives of General Psychiatry, 67(8), 772–782.
Shinto, A. S., Kamaleshwaran, K. K., Aswathi, K. K., Srinivasan, D., Paranthaman, S., Selvaraj, K., Endumathi, R., Vasanthi, A., & Ramakrishnan, T. C. (2015). Differentiating schizophrenia from bipolar illness on 18F FDG PET CT based on white matter metabolism; an under-utilized parameter. International Journal of Nuclear Medicine Research, 2, 12–18.
Spencer, J., O’Brien, J., Riggs, K., Braddick, O., Atkinson, J., & Wattam-Bell, J. (2000). Motion processing in autism: evidence for a dorsal stream deficiency. Neuroreport, 11(12), 2765–2767.
Stedenhouer, J., & Kushner, S. A. (2017). Myelination of parvalbumin interneurons: a parsimonious locus of pathophysiological convergence in schizophrenia. Molecular Psychiatry, 22(1), 4–12.
Stigler, K. A., Sweeten, T. L., Posey, D. J., & McDougle, C. J. (2009). Autism and immune factors: a comprehensive review. Research in Autism Spectrum Disorders, 3(4), 840–860.
Suzuki, K., Sugihara, G., Ouchi, Y., Nakamura, K., Futatsubashi, M., Takebayashi, K., Yoshihara, Y., Omata, K., Matsumoto, K., Tsuchiya, K. J., Sugiyama, T., & Mori, N. (2013). Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry, 70(1), 49–58.
Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers.
Tohka, J., Krestyannikov, E., Dinov, I. D., Graham, A. M., Shattuck, D. W., Ruotsalainen, U., & Toga, A. W. (2007). Genetic algorithms for finite mixture model based voxel classification in neuroimaging. IEEE Transactions on Medical Imaging, 26(5), 696–711.
Torigian, D. A., Green-McKenzie, J., Liu, X., Shofer, F. S., Werner, T., Smith, C. E., Strasser, A. A., Moghbel, M. C., Parekh, A. H., Choi, G., Goncalves, M. D., Spaccarelli, N., Gholami, S., Kumar, P. S., Udupa, J. K., Mesaros, C., & Alavi, A. (2017). A study of the feasibility of FDG-PET/CT to systematically detect and quantify differential metabolic effects of chronic tobacco use in organs of the whole body: a prospective pilot study. Academic Radiology, 24(8), 930–940.
Travers, B. G., Adluru, N., Ennis, C., Tromp, D. P. M., Destiche, D., Doran, S., Bigler, E. D., Lange, N., Lainhart, J. E., & Alexander, A. L. (2012). Diffusion tensor imaging in autism spectrum disorder: a review. Autism Research, 5(5), 289–313.
van den Heuvel, M. P., & Fornito, A. (2014). Brain networks in schizophrenia. Neuropsychology Review, 24(1), 32–48.
Volkow, N. D., Wang, G. J., Shokri Kojori, E., Fowler, J. S., Benveniste, H., & Tomasi, D. (2015). Alcohol decreases baseline brain glucose metabolism more in heavy drinkers than controls but has no effect on stimulation-induced metabolic increases. Journal of Neuroscience, 35(7), 3248–3255.
Weber, B., Fouad, K., Burger, C., & Buck, A. (2002). White matter glucose metabolism during intracortical electrostimulation: a quantitative [18F]fluorodeoxyglucose autoradiography study in the rat. NeuroImage, 16, 993–998.
Woods, R. P., Mazziotta, J. C., & Cherry, S. R. (1993). MRI-PET registration with automated algorithm. Journal of Computer Assisted Tomography, 17, 536–546.
Wright, S. N., Hong, L. E., Winkler, A. M., Chiappelli, J., Nugent, K., Muellerklein, F., Du, X., Rowland, L. M., Wang, D. J. J., & Kochunov, P. (2015). Perfusion shift from white to gray matter may account for processing speed deficits in schizophrenia. Human Brain Mapping, 36(10), 3793–3804.
Yamasaki, T., Maekawa, T., Miyanaga, Y., Takahashi, K., Takamia, N., Ogata, K., & Tobimatsu, S. (2017). Enhanced fine-form perception does not contribute to gestalt face perception in autism spectrum disorder. PLoS One, 12(2), e0170239.
Acknowledgements
This work was partly supported by NARSAD Young Investigator Award and NIMH MH 077146 grant to Serge A. Mitelman and by NIMH grants P50 MH 66392-01, MH 60023, and MH 56489 to Monte S. Buchsbaum.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in this study were in accordance with the ethical standards of the Mount Sinai institutional research committee, as well as with the 1964 Helsinki declaration and its later amendments. The project was approved by the institutional review board of The Icahn School of Medicine at Mount Sinai. Informed consent was obtained from all individual participants in the study.
Conflict of interest
Author Serge A. Mitelman declares that he has no conflict of interest to report. Author Marie-Cecile Bralet declares that she has no conflict of interest to report. Author Derek S. Young declares that he has no conflict of interest to report. Author M. Mehmet Haznedar declares that he has no conflict of interest to report. Author Eric Hollander has received consultation fees from Transceit, Neuropharm, and Nastech. Author Lina Shihabuddin declares that she has no conflict of interest to report. Author Erin A. Hazlett declares that she has no conflict of interest to report. Author Monte S. Buchsbaum declares that he has no conflict of interest to report.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mitelman, S.A., Buchsbaum, M.S., Young, D.S. et al. Increased white matter metabolic rates in autism spectrum disorder and schizophrenia. Brain Imaging and Behavior 12, 1290–1305 (2018). https://doi.org/10.1007/s11682-017-9785-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-017-9785-9