Abstract
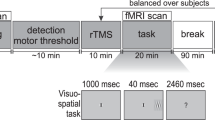
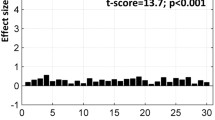
Because the visual cortices are contra-laterally organized, inter-hemispheric transfer tasks have been used to behaviorally probe how information briefly presented to one hemisphere of the visual cortex is integrated with responses resulting from the ipsi- or contra-lateral motor cortex. By forcing rapid information exchange across diverse regions, these tasks robustly activate not only gray matter regions, but also white matter tracts. It is likely that the response hand itself (dominant or non-dominant) modulates gray and white matter activations during within and inter-hemispheric transfer. Yet the role of uni-manual responses and/or right hand dominance in modulating brain activations during such basic tasks is unclear. Here we investigated how uni-manual responses with either hand modulated activations during a basic visuo-motor task (the established Poffenberger paradigm) alternating between inter- and within-hemispheric transfer conditions. In a large sample of strongly right-handed adults (n = 49), we used a factorial combination of transfer condition [Inter vs. Within] and response hand [Dominant(Right) vs. Non-Dominant (Left)] to discover fMRI-based activations in gray matter, and in narrowly defined white matter tracts. These tracts were identified using a priori probabilistic white matter atlases. Uni-manual responses with the right hand strongly modulated activations in gray matter, and notably in white matter. Furthermore, when responding with the left hand, activations during inter-hemispheric transfer were strongly predicted by the degree of right-hand dominance, with increased right-handedness predicting decreased fMRI activation. Finally, increasing age within the middle-aged sample was associated with a decrease in activations. These results provide novel evidence of complex relationships between uni-manual responses in right-handed subjects, and activations during within- and inter-hemispheric transfer suggest that the organization of the motor system exerts sophisticated functional effects. Moreover, our evidence of activation in white matter tracts is consistent with prior studies, confirming fMRI-detectable white matter activations which are systematically modulated by experimental condition.









Similar content being viewed by others
Change history
22 September 2017
The original version of this article unfortunately contained a mistake. The family name of Paolo Brambilla was incorrectly spelled as Bambilla.
References
Aboitiz, F., Lopez, J., & Montiel, J. (2003). Long distance communication in the human brain: Timing constraints for inter-hemispheric synchrony and the origin of brain lateralization. Biological Research, 36(1), 89–99.
Allison, J. D., Meador, K. J., Loring, D. W., Figueroa, R. E., & Wright, J. C. (2000). Functional MRI cerebral activation and deactivation during finger movement. Neurology, 54(1), 135–142.
Amaro Jr., E., & Barker, G. J. (2006). Study design in fMRI: Basic principles. Brain and Cognition, 60(3), 220–232.
Amunts, K., Schlaug, G., Schleicher, A., Steinmetz, H., Dabringhaus, A., Roland, P. E., et al. (1996). Asymmetry in the human motor cortex and handedness. [Research Support, non-U.S. Gov't]. NeuroImage, 4(3 Pt 1), 216–222. doi:10.1006/nimg.1996.0073.
Amunts, K., Jancke, L., Mohlberg, H., Steinmetz, H., & Zilles, K. (2000). Interhemispheric asymmetry of the human motor cortex related to handedness and gender. [Clinical trial Research Support, non-U.S. Gov't]. Neuropsychologia, 38(3), 304–312.
Asemi, A., Ramaseshan, K., Burgess, A., Diwadkar, V. A., & Bressler, S. L. (2015). Dorsal anterior cingulate cortex modulates supplementary motor area in coordinated unimanual motor behavior. Frontiers in Human Neuroscience, 9, 309. doi:10.3389/fnhum.2015.00309.
Ashburner, J., & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26(3), 839–851. doi:10.1016/j.neuroimage.2005.02.018.
Astafiev, S. V., Shulman, G. L., Metcalf, N. V., Rengachary, J., MacDonald, C. L., Harrington, D. L., et al. (2015). Abnormal white matter blood-oxygen-level-dependent signals in chronic mild traumatic brain injury. Journal of Neurotrauma, 32(16), 1254–1271. doi:10.1089/neu.2014.3547.
Bareham, C. A., Bekinschtein, T. A., Scott, S. K., & Manly, T. (2015). Does left-handedness confer resistance to spatial bias? Scientific Reports, 5, 9162. doi:10.1038/srep09162.
Bartolomeo, P., & Thiebaut de Schotten, M. (2016). Let thy left brain know what thy right brain doeth: Inter-hemispheric compensation of functional deficits after brain damage. Neuropsychologia, 93(Pt B), 407–412. doi:10.1016/j.neuropsychologia.2016.06.016.
Berlucchi, G., Aglioti, S., Marzi, C. A., & Tassinari, G. (1995). Corpus Callosum and simple visuomotor integration. [review]. Neuropsychologia, 33(8), 923–936.
Bloom, J. S., & Hynd, G. W. (2005). The role of the corpus callosum in interhemispheric transfer of information: Excitation or inhibition? [Research Support, N.I.H., Extramural review]. Neuropsychology Review, 15(2), 59–71. doi:10.1007/s11065-005-6252-y.
Byrne, M., Clafferty, R. A., Cosway, R., Grant, E., Hodges, A., Lawrie, S. M., et al. (2004). Measurement of lateral preferences and schizophrenia: Results of the Edinburgh high-risk study and methodological issues. [Research Support, non-U.S. Gov't]. Psychiatry Research, 125(3), 205–217. doi:10.1016/j.psychres.2004.01.001.
Dale, A. M. (1999). Optimal experimental design for event-related fMRI. [Research Support, non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] Human Brain Mapping, 8(2–3), 109–114.
Diwadkar, V. A., Bellani, M., Ahmed, R., Dusi, N., Rambaldelli, G., Perlini, C., et al. (2016). Chronological age and its impact on associative learning proficiency and brain structure in middle adulthood. Behavioural Brain Research, 297, 329–337. doi:10.1016/j.bbr.2015.10.016.
Diwadkar, V. A., Asemi, A., Burgess, A., Chowdury, A., & Bressler, S. L. (2017). Potentiation of motor sub-networks for motor control but not working memory: Interaction of dACC and SMA revealed by resting-state directed functional connectivity. PloS One, 12(3), e0172531. doi:10.1371/journal.pone.0172531.
Dragovic, M. (2004). Categorization and validation of handedness using latent class analyses. Acta Neuropsychiatrica, 16, 212–218.
Dreher, J. C., & Grafman, J. (2002). The roles of the cerebellum and basal ganglia in timing and error prediction. The European Journal of Neuroscience, 16(8), 1609–1619.
Eacott, M. J., & Gaffan, D. (1990). Interhemispheric transfer of visuomotor conditional learning via the anterior corpus callosum of monkeys. [Research Support, non-U.S. Gov't]. Behavioural Brain Research, 38(2), 109–116.
Fling, B. W., & Seidler, R. D. (2012). Fundamental differences in callosal structure, neurophysiologic function, and bimanual control in young and older adults. [Comparative study Research Support, N.I.H., Extramural]. Cerebral Cortex, 22(11), 2643–2652. doi:10.1093/cercor/bhr349.
Friedman, A., Burgess, A., Ramaseshan, K., Easter, P., Khatib, D., Chowdury, A., et al. (2017). Brain network dysfunction in obsessive-compulsive disorder induced by simple uni-manual behavior: The role of the dorsal anterior cingulate cortex. Psychiatry Research: Neuroimaging, 260, 6–15.
Friston, K. J., Holmes, A. P., Worsely, K. J., Poline, J. B., Frith, C. D., & Frackowiak, R. S. J. (1995). Statistical parametric maps in functional imaging: A general approach. Human Brain Mapping, 2, 189–210.
Gawryluk, J. R., Brewer, K. D., Beyea, S. D., & D'Arcy, R. C. (2009). Optimizing the detection of white matter fMRI using asymmetric spin echo spiral. [evaluation studies Research Support, non-U.S. Gov't]. NeuroImage, 45(1), 83–88. doi:10.1016/j.neuroimage.2008.11.005.
Gawryluk, J. R., D'Arcy, R. C., Mazerolle, E. L., Brewer, K. D., & Beyea, S. D. (2011a). Functional mapping in the corpus callosum: A 4T fMRI study of white matter. [Research Support, non-U.S. Gov't]. NeuroImage, 54(1), 10–15. doi:10.1016/j.neuroimage.2010.07.028.
Gawryluk, J. R., Mazerolle, E. L., Brewer, K. D., Beyea, S. D., & D'Arcy, R. C. (2011b). Investigation of fMRI activation in the internal capsule. [Research Support, non-U.S. Gov't]. BMC Neurosci, 12, 56. doi:10.1186/1471-2202-12-56.
Gawryluk, J. R., Mazerolle, E. L., & D'Arcy, R. C. (2014). Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. [review]. Front Neurosci, 8, 239. doi:10.3389/fnins.2014.00239.
Gazzaniga, M. S. (1989). Organization of the human brain. Science, 245(4921), 947–952.
Gazzaniga, M. S. (2000). Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain, 123(Pt 7), 1293–1326.
Geffen, G. M., Jones, D. L., & Geffen, L. B. (1994). Interhemispheric control of manual motor activity. [Research Support, non-U.S. Gov't review]. Behavioural Brain Research, 64(1–2), 131–140.
Grecucci, A., Crescentini, C., & Siugzdaite, R. (2008). Vicarious function in the motor cortex. A computational investigation. Neuroscience Letters, 434(2), 185–190. doi:10.1016/j.neulet.2008.01.059.
Grefkes, C., Eickhoff, S. B., Nowak, D. A., Dafotakis, M., & Fink, G. R. (2008). Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. NeuroImage, 41(4), 1382–1394. doi:10.1016/j.neuroimage.2008.03.048.
Haber, S. N., & Calzavara, R. (2009). The cortico-basal ganglia integrative network: The role of the thalamus. Brain Research Bulletin, 78(2–3), 69–74. doi:10.1016/j.brainresbull.2008.09.013.
Hayashi, M. J., Saito, D. N., Aramaki, Y., Asai, T., Fujibayashi, Y., & Sadato, N. (2008). Hemispheric asymmetry of frequency-dependent suppression in the ipsilateral primary motor cortex during finger movement: A functional magnetic resonance imaging study. [Research Support, non-U.S. Gov't]. Cerebral Cortex, 18(12), 2932–2940. doi:10.1093/cercor/bhn053.
Hellige, J. B. (1996). Hemispheric asymmetry for visual information processing. [Research Support, U.S. Gov't, non-P.H.S. Review]. Acta Neurobiologiae Experimentalis (Wars), 56(1), 485–497.
Herrero, M. T., Barcia, C., & Navarro, J. M. (2002). Functional anatomy of thalamus and basal ganglia. Child's Nervous System, 18(8), 386–404. doi:10.1007/s00381-002-0604-1.
Hua, K., Zhang, J., Wakana, S., Jiang, H., Li, X., Reich, D. S., et al. (2008). Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. [Research Support, N.I.H., Extramural Research Support, non-U.S. Gov't]. NeuroImage, 39(1), 336–347. doi:10.1016/j.neuroimage.2007.07.053.
Huang, H., & Ding, M. (2016). Linking functional connectivity and structural connectivity quantitatively: A comparison of methods. Brain Connectivity, 6(2), 99–108. doi:10.1089/brain.2015.0382.
Jagtap, P., & Diwadkar, V. A. (2016). Effective connectivity of ascending and descending frontalthalamic pathways during sustained attention: Complex brain network interactions in adolescence. Human Brain Mapping. doi:10.1002/hbm.23196.
Jaillard, A., Martin, C. D., Garambois, K., Lebas, J. F., & Hommel, M. (2005). Vicarious function within the human primary motor cortex? A longitudinal fMRI stroke study. [Research Support, non-U.S. Gov't]. Brain, 128(Pt 5), 1122–1138. doi:10.1093/brain/awh456.
Jeannerod, M. (1988). The neural and behavioural organization of goal directed movements. Oxford: Oxford University Press.
Josephs, O., & Henson, R. N. (1999). Event-related functional magnetic resonance imaging: Modelling, inference and optimization. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 354(1387), 1215–1228.
Kawashima, R., Okuda, J., Umetsu, A., Sugiura, M., Inoue, K., Suzuki, K., et al. (2000). Human cerebellum plays an important role in memory-timed finger movement: An fMRI study. Journal of Neurophysiology, 83(2), 1079–1087.
Kim, S. G., Ashe, J., Hendrich, K., Ellermann, J. M., Merkle, H., Ugurbil, K., et al. (1993). Functional magnetic resonance imaging of motor cortex: Hemispheric asymmetry and handedness. [Research Support, non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] Science, 261(5121), 615–617.
Kobayashi, M., Hutchinson, S., Schlaug, G., & Pascual-Leone, A. (2003). Ipsilateral motor cortex activation on functional magnetic resonance imaging during unilateral hand movements is related to interhemispheric interactions. [Research Support, non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] NeuroImage, 20(4), 2259–2270.
Lin, F. H., Agnew, J. A., Belliveau, J. W., & Zeffiro, T. A. (2009). Functional and effective connectivity of visuomotor control systems demonstrated using generalized partial least squares and structural equation modeling. [Research Support, N.I.H., Extramural Research Support, non-U.S. Gov't]. Human Brain Mapping, 30(7), 2232–2251. doi:10.1002/hbm.20664.
Liu, T. T. (2004). Efficiency, power, and entropy in event-related fMRI with multiple trial types. Part II: Design of experiments. [Comparative study Research Support, non-U.S. Gov't Research Support, U.S. Gov't, non-P.H.S.] NeuroImage, 21(1), 401–413.
Logothetis, N. K. (2008). What we can do and what we cannot do with fMRI. Nature, 453(7197), 869–878. doi:10.1038/nature06976.
Logothetis, N. K., & Wandell, B. A. (2004). Interpreting the BOLD signal. Annual Review of Physiology, 66, 735–769.
Maldjian, J. A., Laurienti, P. J., Kraft, R. A., & Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239.
Martuzzi, R., Murray, M. M., Maeder, P. P., Fornari, E., Thiran, J., Clarke, S., et al. (2006). Visuo-motor pathways in humans revealed by event-related fMRI. [Research Support, non-U.S. Gov't]. Experimental Brain Research, 170(4), 472–487. doi:10.1007/s00221-005-0232-6.
Marzi, C. A. (1999). The Poffenberger paradigm: A first, simple, behavioural tool to study interhemispheric transmission in humans. [biography historical article]. Brain Research Bulletin, 50(5–6), 421–422.
Marzi, C. A., Bisiacchi, P., & Nicoletti, R. (1991). Is interhemispheric transfer of visuomotor information asymmetric? Evidence from a meta-analysis. [meta-analysis]. Neuropsychologia, 29(12), 1163–1177.
Mazerolle, E. L., D'Arcy, R. C., & Beyea, S. D. (2008). Detecting functional magnetic resonance imaging activation in white matter: interhemispheric transfer across the corpus callosum. [Clinical Trial Research Support, Non-U.S. Gov't]. BMC Neurosci, 9, 84. doi:10.1186/1471-2202-9-84.
Mazerolle, E. L., Beyea, S. D., Gawryluk, J. R., Brewer, K. D., Bowen, C. V., & D'Arcy, R. C. (2010). Confirming white matter fMRI activation in the corpus callosum: Co-localization with DTI tractography. [Research Support, non-U.S. Gov't]. NeuroImage, 50(2), 616–621. doi:10.1016/j.neuroimage.2009.12.102.
McKay, N. S., Iwabuchi, S. J., Haberling, I. S., Corballis, M. C., & Kirk, I. J. (2017). Atypical white matter microstructure in left-handed individuals. Laterality, 22(3), 257–267. doi:10.1080/1357650X.2016.1175469.
Mooshagian, E., Iacoboni, M., & Zaidel, E. (2009). Spatial attention and interhemispheric visuomotor integration in the absence of the corpus callosum. [case Reports Research Support, N.I.H., Extramural]. Neuropsychologia, 47(3), 933–937. doi:10.1016/j.neuropsychologia.2008.12.005.
Nachev, P., Wydell, H., O'Neill, K., Husain, M., & Kennard, C. (2007). The role of the pre-supplementary motor area in the control of action. [case Reports Research Support, non-U.S. Gov't]. Neuroimage, 36(Suppl 2), T155–T163. doi:10.1016/j.neuroimage.2007.03.034.
Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113.
Park, H. J., & Friston, K. (2013). Structural and functional brain networks: From connections to cognition. [Research Support, non-U.S. Gov't review]. Science, 342(6158), 1238411. doi:10.1126/science.1238411.
Poffenberger, A. T. (1912). Reaction time to retinal stimulation with special reference to the time lost in conduction through nervous centers. Archiv für Psychologie, 23, 1–73.
Price, C. J., & Friston, K. J. (2005). Functional ontologies for cognition: The systematic definition of structure and function. Cognitive Neuropsychology, 22(3), 262–275. doi:10.1080/02643290442000095.
Raz, N., & Lindenberger, U. (2011). Only time will tell: Cross-sectional studies offer no solution to the age-brain-cognition triangle: Comment on Salthouse (2011). [comment Research Support, N.I.H., Extramural]. Psychological Bulletin, 137(5), 790–795. doi:10.1037/a0024503.
Savazzi, S., Fabri, M., Rubboli, G., Paggi, A., Tassinari, C. A., & Marzi, C. A. (2007). Interhemispheric transfer following callosotomy in humans: Role of the superior colliculus. [Research Support, non-U.S. Gov't]. Neuropsychologia, 45(11), 2417–2427. doi:10.1016/j.neuropsychologia.2007.04.002.
Schulte, T., & Muller-Oehring, E. M. (2010). Contribution of callosal connections to the interhemispheric integration of visuomotor and cognitive processes. [Research Support, N.I.H., Extramural review]. Neuropsychology Review, 20(2), 174–190. doi:10.1007/s11065-010-9130-1.
Schulte, T., Sullivan, E. V., Muller-Oehring, E. M., Adalsteinsson, E., & Pfefferbaum, A. (2005). Corpus Callosal microstructural integrity influences interhemispheric processing: A diffusion tensor imaging study. Cereb Cortex.
Serbruyns, L., Leunissen, I., van Ruitenbeek, P., Pauwels, L., Caeyenberghs, K., Solesio-Jofre, E., et al. (2016). Alterations in brain white matter contributing to age-related slowing of task switching performance: The role of radial diffusivity and magnetization transfer ratio. Human Brain Mapping, 37(11), 4084–4098. doi:10.1002/hbm.23297.
Serrien, D. J., Ivry, R. B., & Swinnen, S. P. (2006). Dynamics of hemispheric specialization and integration in the context of motor control. [Research Support, N.I.H., Extramural Research Support, non-U.S. Gov't]. Nature Reviews. Neuroscience, 7(2), 160–166. doi:10.1038/nrn1849.
Stephan, K. E., Marshall, J. C., Penny, W. D., Friston, K. J., & Fink, G. R. (2007). Interhemispheric integration of visual processing during task-driven lateralization. The Journal of Neuroscience, 27(13), 3512–3522. doi:10.1523/JNEUROSCI.4766-06.2007.
Stroman, P. W., & Ryner, L. N. (2001). Functional MRI of motor and sensory activation in the human spinal cord. Magnetic Resonance Imaging, 19(1), 27–32.
Tettamanti, M., Paulesu, E., Scifo, P., Maravita, A., Fazio, F., Perani, D., et al. (2002). Interhemispheric transmission of visuomotor information in humans: fMRI evidence. Journal of Neurophysiology, 88(2), 1051–1058.
Toga, A. W., & Thompson, P. M. (2003). Mapping brain asymmetry. [Research Support, U.S. Gov't, P.H.S. Review]. Nature Reviews. Neuroscience, 4(1), 37–48. doi:10.1038/nrn1009.
Tootell, R. B., Mendola, J. D., Hadjikhani, N. K., Liu, A. K., & Dale, A. M. (1998). The representation of the ipsilateral visual field in human cerebral cortex. [Research Support, non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. Review]. Proceedings of the National Academy of Sciences of the United States of America, 95(3), 818–824.
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289.
Tzourio-Mazoyer, N., Petit, L., Zago, L., Crivello, F., Vinuesa, N., Joliot, M., et al. (2015). Between-hand difference in ipsilateral deactivation is associated with hand lateralization: fMRI mapping of 284 volunteers balanced for handedness. Frontiers in Human Neuroscience, 9, 5. doi:10.3389/fnhum.2015.00005.
van den Berg, F. E., Swinnen, S. P., & Wenderoth, N. (2011). Involvement of the primary motor cortex in controlling movements executed with the ipsilateral hand differs between left- and right-handers. [Research Support, non-U.S. Gov't]. Journal of Cognitive Neuroscience, 23(11), 3456–3469. doi:10.1162/jocn_a_00018.
Ward, B. D. (2000). Simultaneous inference for fMRI data. Milwaukee, WI: Medical College of Wisconsin.
Weber, B., Treyer, V., Oberholzer, N., Jaermann, T., Boesiger, P., Brugger, P., et al. (2005). Attention and interhemispheric transfer: A behavioral and fMRI study. [Comparative study]. Journal of Cognitive Neuroscience, 17(1), 113–123. doi:10.1162/0898929052880002.
Yalachkov, Y., Kaiser, J., Doehrmann, O., & Naumer, M. J. (2015). Enhanced visuo-haptic integration for the non-dominant hand. [Research Support, non-U.S. Gov't]. Brain Research, 1614, 75–85. doi:10.1016/j.brainres.2015.04.020.
Yarkoni, T., Barch, D. M., Gray, J. R., Conturo, T. E., & Braver, T. S. (2009). BOLD correlates of trial-by-trial reaction time variability in gray and white matter: A multi-study fMRI analysis. PloS One, 4(1), e4257. doi:10.1371/journal.pone.0004257.
Zaidel, E., & Iacoboni, M. (Eds.). (2003). The parallel brain: The cognitive neuroscience of the corpus callosum. Cambridge: MIT Press.
Zhang, Y., Zhang, J., Oishi, K., Faria, A. V., Jiang, H., Li, X., et al. (2010). Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. [Research Support, N.I.H., Extramural]. NeuroImage, 52(4), 1289–1301. doi:10.1016/j.neuroimage.2010.05.049.
Acknowledgements
This study was partly supported from grants from Fondazione CariVerona (Obiettivo di ricerca A9 del progetto di Neuroscience "Disabilità cognitiva e comportamentale nelle demenze e nelle psicosi”) to PB and MR and from the Ministry of Health to PB (GR-2010-2319022), and MB (GR-2010-2316745). VAD was supported by a Cooperint Visiting Professorship from the University of Verona, a Career Development Chair from Wayne State University, the Thomas Gershenson Distinguished Faculty Fellowship from Wayne State University, the Lyckaki-Young Fund from the State of Michigan, the Prechter Family Bipolar Foundation, the Children’s Hospital of Michigan Foundation, the Cohen Neuroscience Endowmentand the National Institute of Mental Health (MH111177). We thank Alberto Beltramello for helping in MRI acquisition and data management. The work constitutes a collaborative effort between VAD and PB. Their authorship order is inter-changeable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to report.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
Additional information
The original version of this article was revised: The family name of Paolo Brambilla was corrected
Rights and permissions
About this article
Cite this article
Diwadkar, V.A., Bellani, M., Chowdury, A. et al. Activations in gray and white matter are modulated by uni-manual responses during within and inter-hemispheric transfer: effects of response hand and right-handedness. Brain Imaging and Behavior 12, 942–961 (2018). https://doi.org/10.1007/s11682-017-9750-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-017-9750-7