Abstract
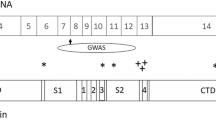
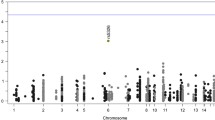
GRM5 (coding for metabotropic glutamate receptor 5, mGluR5) is a promising target for the treatment of cognitive deficits in schizophrenia, but there has been little investigation of its association with cognitive and brain phenotypes within this disorder. We examined the effects of common genetic variation in GRM5 with cognitive function, hippocampal volume, and hippocampal mGluR5 protein levels in schizophrenia patients relative to healthy controls. Two independent GRM5 variants rs60954128 [C>T] and rs3824927 [G>T] were genotyped in a schizophrenia case/control cohort (n=249/261). High-resolution anatomical brain scans were available for a subset of the cohort (n=103 schizophrenia /78 control). All participants completed a standard set of neuropsychological tests. In a separate postmortem cohort (n=19 schizophrenia/20 controls), hippocampal mGluR5 protein levels were examined among individuals of different GRM5 genotypes. Schizophrenia minor allele carriers of rs60954128 had reduced right hippocampal volume relative to healthy controls of the same genotype (−12.3%); this effect was exaggerated in males with schizophrenia (−15.6%). For rs3824927, compared to major allele homozygotes, minor allele carriers with schizophrenia had lower Intelligence Quotients (IQ). Examination in hippocampal postmortem tissue showed no difference in mGluR5 protein expression according to genotype for either rs60954128 or rs3824927. While these genetic variants in GRM5 were associated with cognitive impairments and right hippocampal volume reduction in schizophrenia, they did not affect protein expression. Further study of these mechanisms may help to delineate new targets for the treatment of cognitive deficits in schizophrenia, and may be relevant to other disorders.



Similar content being viewed by others
References
Aickin, M., & Gensler, H. (1996). Adjusting for multiple testing when reporting research results: The Bonferroni vs Holm methods. American Journal of Public Health, 86(5), 726–728.
Attucci, S., Carla, V., Mannaioni, G., & Moroni, F. (2001). Activation of type 5 metabotropic glutamate receptors enhances NMDA responses in mice cortical wedges. British Journal of Pharmacology, 132(4), 799–806.
Ayala, J. E., Chen, Y., Banko, J. L., Sheffler, D. J., Williams, R., Telk, A. N., et al. (2009). mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology, 34(9), 2057–2071. doi:10.1038/npp.2009.30.
Ayalew, M., Le-Niculescu, H., Levey, D. F., Jain, N., Changala, B., Patel, S. D., et al. (2012). Convergent functional genomics of schizophrenia: From comprehensive understanding to genetic risk prediction. Molecular Psychiatry, 17(9), 887–905.
Beck, K., Javitt, D. C., & Howes, O. D. (2016). Targeting glutamate to treat schizophrenia: Lessons from recent clinical studies. Psychopharmacology, 233(13), 2425–2428.
Colom, R., Stein, J. L., Rajagopalan, P., Martínez, K., Hermel, D., Wang, Y., et al. (2013). Hippocampal structure and human cognition: Key role of spatial processing and evidence supporting the efficiency hypothesis in females. Intelligence, 41(2), 129–140.
Devon, Anderson S., Teague, P., Muir, W., Murray, V., Pelosi, A., et al. (2001). The genomic organisation of the metabotropic glutamate receptor subtype 5 gene, and its association with schizophrenia. Molecular Psychiatry, 6(3), 311.
Doherty, J. L., & Owen, M. J. (2014). Genomic insights into the overlap between psychiatric disorders: Implications for research and clinical practice. Genome Medicine, 6(4), 29. doi:10.1186/gm546.
Dölen, G., & Bear, M. F. (2008). Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. The Journal of Physiology, 586(6), 1503–1508.
Elia, J., Gai, X., Xie, H. M., Perin, J. C., Geiger, E., Glessner, J. T., et al. (2010). Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Molecular Psychiatry, 15(6), 637–646.
Goldstein, J. M., Cherkerzian, S., Tsuang, M. T., & Petryshen, T. L. (2013). Sex differences in the genetic risk for schizophrenia: History of the evidence for sex-specific and sex-dependent effects. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 162(7), 698–710.
Jia, Z., Lu, Y., Henderson, J., Taverna, F., Romano, C., Abramow-Newerly, W., et al. (1998). Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learning & Memory, 5(4), 331–343. doi:10.1101/lm.5.4.331.
Kirov, G., Pocklington, A. J., Holmans, P., Ivanov, D., Ikeda, M., Ruderfer, D., et al. (2012). De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Molecular Psychiatry, 17(2), 142–153. doi:10.1038/mp.2011.154.
Krijger, P.H.L., & de Laat, W. (2016). Regulation of disease-associated gene expression in the 3D genome. Nature Reviews Molecular Cell Biology. http://www.nature.com/nrm/journal/vaop/ncurrent/full/nrm.2016.138.html. Accessed 24 January 2017.
Kuntsi, J., Eley, T. C., Taylor, A., Hughes, C., Asherson, P., Caspi, A., & Moffitt, T. E. (2004). Co-occurrence of ADHD and low IQ has genetic origins. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 124(1), 41–47.
Loughland, C., Draganic, D., McCabe, K., Richards, J., Nasir, A., Allen, J., et al. (2010). Australian schizophrenia research Bank: A database of comprehensive clinical, endophenotypic and genetic data for aetiological studies of schizophrenia. Australian and New Zealand Journal of Psychiatry, 44(11), 1029–1035.
Malhotra, A. K., Pinals, D. A., Weingartner, H., Sirocco, K., Missar, C. D., Pickar, D., & Breier, A. (1996). NMDA receptor function and human cognition: The effects of ketamine in healthy volunteers. Neuropsychopharmacology, 14(5), 301–307. doi:10.1016/0893-133x(95)00137-3.
Manahan-Vaughan, D., & Braunewell, K. H. (2005). The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cerebral Cortex, 15(11), 1703–1713.
Mannaioni, G., Marino, M. J., Valenti, O., Traynelis, S. F., & Conn, P. J. (2001). Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. The Journal of Neuroscience, 21(16), 5925–5934.
Matosin, N., Fernandez-Enright, F., Lum, J. S., Andrews, J. L., Engel, M., Huang, X.-F., & Newell, K. A. (2015a). Metabotropic glutamate receptor 5, and its trafficking molecules Norbin and Tamalin, are increased in the CA1 hippocampal region of subjects with schizophrenia. Schizophrenia Research, 166(1–3), 212–218. doi:10.1016/j.schres.2015.05.001.
Matosin, N., Fernandez-Enright, F., Lum, J. S., & Newell, K. A. (2015b). Shifting towards a model of mGluR5 dysregulation in schizophrenia: Consequences for future schizophrenia treatment. Neuropharmacology. doi:10.1016/j.neuropharm.2015.08.003.
Millar, J. K., Brown, J., Maule, J. C., Shibasaki, Y., Christie, S., Lawson, D., et al. (1998). A long-range restriction map across 3 Mb of the chromosome 11 breakpoint region of a translotion linked to schizophrenia: Localization of the breakpoint and the search for neighbouring genes. Psychiatric Genetics, 8(3), 175–182.
Minh, T. N. N. (2014). Relationship between subcortical volumes and cognitive abilities in young healthy adults. Vietnam: International University HCMC Retrieved from http://csc.hcmiu.edu.vn:8080/dspace/handle/123456789/1166.
Mukherjee, S., & Manahan-Vaughan, D. (2013). Role of metabotropic glutamate receptors in persistent forms of hippocampal plasticity and learning. Neuropharmacology, 66, 65–81.
Randolph, C. (1998). RBANS manual: Repeatable battery for the assessment of neuropsychological status. San Antonio, TX: The Psychological Corporation.
Ripke, S., O’Dushlaine, C., Chambert, K., Moran, J. L., Kähler, A. K., Akterin, S., et al. (2013). Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nature Genetics, 45(10), 1150–1159. doi:10.1038/ng.2742.
Ripke, S., Sanders, A. R., Kendler, K. S., Levinson, D. F., Sklar, P., Holmans, P. A., et al. (2011). Genome-wide association study identifies five new schizophrenia loci. Nature Genetics, 43(10), 969–976. doi:10.1038/ng.940.
Schizophrenia Working Group of the Psychiatric Genomics Consortium. (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature, 511(7510), 421–427.
Sherry, S. T., Ward, M.-H., Kholodov, M., Baker, J., Phan, L., Smigielski, E. M., & Sirotkin, K. (2001). dbSNP: The NCBI database of genetic variation. Nucleic Acids Research, 29(1), 308–311.
Spreen, O. (1998). A compendium of neuropsychological tests: Administration, norms, and commentary. New York: Oxford University Press.
Sullivan, K., Hatton, D., Hammer, J., Sideris, J., Hooper, S., Ornstein, P., & Bailey, D. (2006). ADHD symptoms in children with FXS. American Journal of Medical Genetics Part A, 140(21), 2275–2288.
Tabor, H. K., Risch, N. J., & Myers, R. M. (2002). Candidate-gene approaches for studying complex genetic traits: Practical considerations. Nature Reviews. Genetics, 3(5), 391–397. doi:10.1038/nrg796.
Timms, A. E., Dorschner, M. O., Wechsler, J., Choi, K. Y., Kirkwood, R., Girirajan, S., et al. (2013). Support for the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia from exome sequencing in multiplex families. JAMA psychiatry (Chicago, Ill.), 70(6), 582–590. doi:10.1001/jamapsychiatry.2013.1195.
Tsai, G., Van Kammen, D. P., Chen, S., Kelley, M. E., Grier, A., & Coyle, J. T. (1998). Glutamatergic neurotransmission involves structural and clinical deficits of schizophrenia. Biological Psychiatry, 44(8), 667–674.
Wechsler, D. (1997). WAIS-III: Administration and scoring manual: Wechsler adult intelligence scale. Psychological Corporation.
Wechsler, D. (2001). Wechsler test of adult reading: WTAR. Psychological Corporation.
Wilkening, S., Chen, B., Bermejo, J. L., & Canzian, F. (2009). Is there still a need for candidate gene approaches in the era of genome-wide association studies? Genomics, 93(5), 415–419.
Yang, Y. S., Marder, S. R., & Green, M. F. (2016). Repurposing drugs for cognition in schizophrenia. Clinical Pharmacology & Therapeutics, n/a-n/a. doi:10.1002/cpt.529
Acknowledgements
This study was supported by a Grant-in-Aid held by Authors NM, KN and FF and the Daniel Beck Award held by Author NM, from the Schizophrenia Research Institute. The study used samples and data from the Australian Schizophrenia Research Bank (ASRB), funded by the Australian National Health and Medical Research Council (NHMRC) Enabling Grant (No. 386500) held by V. Carr, U. Schall, R. Scott, A. Jablensky, B. Mowry, P. Michie, S. Catts, F. Henskens and C. Pantelis (Chief Investigators), and the Pratt Foundation, Ramsay Health Care, the Viertel Charitable Foundation, as well the Schizophrenia Research Institute, using an infrastructure grant from the NSW Ministry of Health. Authors NM and JA were supported by Ian Scott Scholarships from Australian Rotary Health. Author NT was supported by an Australian Postgraduate Award. Author MG was supported by an Australian National Health and Medical Research Council (NHMRC) Biomedical Career Development Fellowship (1061875). Postmortem brain tissues were received from the NSW Tissue Resource Centre, which is supported by the National Health and Medical Research Council of Australia, Schizophrenia Research Institute and the National Institute of Alcohol Abuse and Alcoholism [NIH (NIAA) R24AA012725]. The authors wish to thank P. Bitter and B. Yamasani from the Australian Cancer Research Foundation at The Garvan Institute of Medical Research, Sydney for their assistance with the MassARRAY genotyping assays, as well as A.M. Shepherd and I.C. Gould for assistance with structural imaging and Freesurfer processing. All authors report no financial relationships with commercial interests.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflicts of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study
Additional information
Melissa Jayne Green and Francesca Fernandez contributed equally to this work.
Electronic Supplementary Material
ESM 1
(PDF 167 KB)
Rights and permissions
About this article
Cite this article
Matosin, N., Newell, K.A., Quidé, Y. et al. Effects of common GRM5 genetic variants on cognition, hippocampal volume and mGluR5 protein levels in schizophrenia. Brain Imaging and Behavior 12, 509–517 (2018). https://doi.org/10.1007/s11682-017-9712-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-017-9712-0