Abstract
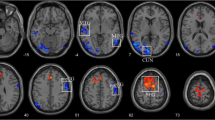
To explore the alterations of functional connectivity (FC) and connections within and between the subnetworks of the visual network and the default mode network in glaucoma. We applied the independent component analysis to obtain two resting-state networks (RSNs), which were the visual network and the default mode network (DMN), from the resting-state fMRI data of 25 primary open-angle glaucoma (POAG) patients and 25 well-matched normal controls. Then FC analysis was performed to obtain the altered FC within the RSNs, whereas the functional network connectivity (FNC) analysis was performed within and between these two RSNs. The abnormalities were correlated with clinical measures in glaucoma to investigate the abnormality-clinical relationship. FC analysis showed that the FC in the occipital pole of the visual network was decreased in POAG patients while no alterations were found in the FC of the DMN in patients. FNC analysis of connections within the RSNs found that two of the three connections within the visual network were decreased while no connection alterations were found within the DMN. FNC analysis of connections between these two RSNs found two increased connections and one decreased connection. The decreased connection between these two RSNs was positively correlated with the visual field mean deviation. These findings shed light on the importance of the reorganization of resting state networks in glaucoma mechanism, which may facilitate the understanding of glaucoma.





Similar content being viewed by others
Notes
Practically, more emphasis should be put on visual field MD and CDR than RNFL.
References
Alba, C., Vidal, L., Diaz, F., Villena, A., & de Vargas, I. P. (2004). Ultrastructural and quantitative age-related changes in capillaries of the dorsal lateral geniculate nucleus. Brain Research Bulletin, 64(2), 145–153. doi:10.1016/j.brainresbull.2004.06.006.
Baird, B., Smallwood, J., Mrazek, M. D., Kam, J. W., Franklin, M. S., & Schooler, J. W. (2012). Inspired by distraction: mind wandering facilitates creative incubation. Psychological Science, 23(10), 1117–1122. doi:10.1177/0956797612446024.
Bell, A. J., & Sejnowski, T. J. (1995). An information maximization approach to blind separation and blind deconvolution. Neural Computation, 7(6), 1129–1159. doi:10.1162/Neco.1995.7.6.1129.
Bola, M., & Sabel, B. A. (2015). Dynamic reorganization of brain functional networks during cognition. NeuroImage, 114, 398–413. doi:10.1016/j.neuroimage.2015.03.057.
Bola, M., Gall, C., Moewes, C., Fedorov, A., Hinrichs, H., & Sabel, B. A. (2014). Brain functional connectivity network breakdown and restoration in blindness. [randomized controlled trial research support, non-U.S. Gov’t]. Neurology, 83(6), 542–551. doi:10.1212/WNL.0000000000000672.
Bola, M., Gall, C., & Sabel, B. A. (2015). Disturbed temporal dynamics of brain synchronization in vision loss. Cortex, 67, 134–146. doi:10.1016/j.cortex.2015.03.020.
Bressler, D. W., Fortenbaugh, F. C., Robertson, L. C., & Silver, M. A. (2013). Visual spatial attention enhances the amplitude of positive and negative fMRI responses to visual stimulation in an eccentricity-dependent manner. Vision Research, 85, 104–112. doi:10.1016/j.visres.2013.03.009.
Calhoun, V. D., Adali, T., Pearlson, G. D., & Pekar, J. J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14(3), 140–151.
Chen, W., Kato, T., Zhu, X. H., Strupp, J., Ogawa, S., & Ugurbil, K. (1998). Mapping of lateral geniculate nucleus activation during visual stimulation in human brain using fMRI. [Clinical Trial Research Support, U.S. Gov’t, P.H.S.]. Magn Reson Med,, 39(1), 89–96.
Chen, W. W., Wang, N., Cai, S., Fang, Z., Yu, M., Wu, Q., et al. (2013). Structural brain abnormalities in patients with primary open-angle glaucoma: a study with 3 T MR imaging. [comparative study research support, non-U.S. Gov’t]. Investigative Ophthalmology & Visual Science, 54(1), 545–554. doi:10.1167/iovs.12-9893.
Dai, H., Mu, K. T., Qi, J. P., Wang, C. Y., Zhu, W. Z., Xia, L. M., et al. (2011). Assessment of lateral geniculate nucleus atrophy with 3 T MR imaging and correlation with clinical stage of glaucoma. [controlled clinical trial]. AJNR. American Journal of Neuroradiology, 32(7), 1347–1353. doi:10.3174/ajnr.A2486.
Dai, H., Morelli, J. N., Ai, F., Yin, D., Hu, C., Xu, D., et al. (2013). Resting-state functional MRI: functional connectivity analysis of the visual cortex in primary open-angle glaucoma patients. [research support, non-U.S. Gov’t]. Human Brain Mapping, 34(10), 2455–2463. doi:10.1002/hbm.22079.
Damoiseaux, J. S., Rombouts, S. A., Barkhof, F., Scheltens, P., Stam, C. J., Smith, S. M., et al. (2006). Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America, 103(37), 13848–13853. doi:10.1073/pnas.0601417103.
Ding, K., Liu, Y., Yan, X., Lin, X., & Jiang, T. (2013). Altered functional connectivity of the primary visual cortex in subjects with amblyopia. Neural Plasticity, 2013, 8. doi:10.1155/2013/612086.
Erhardt, E. B., Rachakonda, S., Bedrick, E. J., Allen, E. A., Adali, T., & Calhoun, V. D. (2011). Comparison of multi-subject ICA methods for analysis of fMRI data. Human Brain Mapping, 32(12), 2075–2095. doi:10.1002/hbm.21170.
Fornito, A., Harrison, B. J., Zalesky, A., & Simons, J. S. (2012). Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proceedings of the National Academy of Sciences of the United States of America, 109(31), 12788–12793. doi:10.1073/pnas.1204185109.
Frezzotti, P., Giorgio, A., Motolese, I., De Leucio, A., Iester, M., Motolese, E., et al. (2014). Structural and functional brain changes beyond visual system in patients with advanced glaucoma. PloS One, 9(8), e105931. doi:10.1371/journal.pone.0105931.
Garaci, F. G., Bolacchi, F., Cerulli, A., Melis, M., Spano, A., Cedrone, C., et al. (2009). Optic nerve and optic radiation neurodegeneration in patients with glaucoma: in vivo analysis with 3-T diffusion-tensor MR imaging. Radiology, 252(2), 496–501. doi:10.1148/radiol.2522081240.
Gentili, C., Ricciardi, E., Gobbini, M. I., Santarelli, M. F., Haxby, J. V., Pietrini, P., et al. (2009). Beyond amygdala: default mode network activity differs between patients with social phobia and healthy controls. Brain Research Bulletin, 79(6), 409–413. doi:10.1016/j.brainresbull.2009.02.002.
Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–258. doi:10.1073/pnas.0135058100.
Gupta, N., & Yucel, Y. H. (2007). Glaucoma as a neurodegenerative disease. Current Opinion in Ophthalmology, 18(2), 110–114. doi:10.1097/Icu.0b013e3280895aea.
Gupta, N., Ang, L. C., de Tilly, L. N., Bidaisee, L., & Yucel, Y. H. (2006a). Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. British Journal of Ophthalmology, 90(6), 674–678. doi:10.1136/bjo.2005.086769.
Jafri, M. J., Pearlson, G. D., Stevens, M., & Calhoun, V. D. (2008). A method for functional network connectivity among spatially independent resting-state components in schizophrenia. NeuroImage, 39(4), 1666–1681. doi:10.1016/j.neuroimage.2007.11.001.
Joel, S. E., Caffo, B. S., Zijl, P. C. M., & Pekar, J. J. (2011). On the relationship between seed-based and ICA-based measures of functional connectivity. Magnetic Resonance in Medicine, 66(3), 644–657. doi:10.1002/mrm.22818.
Lee, L., Harrison, L. M., & Mechelli, A. (2003). A report of the functional connectivity workshop, Dusseldorf 2002. NeuroImage, 19(2 Pt 1), 457–465.
Li, Y. O., Adali, T., & Calhoun, V. D. (2007). Estimating the number of independent components for functional magnetic resonance imaging data. Human Brain Mapping, 28(11), 1251–1266. doi:10.1002/hbm.20359.
Li, T., Liu, Z., Li, J., Liu, Z., Tang, Z., Xie, X., et al. (2015). Altered amplitude of low-frequency fluctuation in primary open-angle glaucoma: a resting-state FMRI study. Investigative Ophthalmology & Visual Science, 56(1), 322–329. doi:10.1167/iovs.14-14974.
Lin, X. M., Ding, K., Liu, Y., Yan, X. H., Song, S. J., & Jiang, T. Z. (2012). Altered spontaneous activity in anisometropic amblyopia subjects: revealed by resting-state fMRI. PloS One, 7(8). doi:10.1371/journal.pone.0043373.
McKeown, M. J., Makeig, S., Brown, G. G., Jung, T. P., Kindermann, S. S., Bell, A. J., et al. (1998). Analysis of fMRI data by blind separation into independent spatial components. Human Brain Mapping, 6(3), 160–188.
Murphy, K., Birn, R. M., Handwerker, D. A., Jones, T. B., & Bandettini, P. A. (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage, 44(3), 893–905. doi:10.1016/j.neuroimage.2008.09.036.
Ongur, D., Lundy, M., Greenhouse, I., Shinn, A. K., Menon, V., Cohen, B. M., et al. (2010). Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Research, 183(1), 59–68. doi:10.1016/j.pscychresns.2010.04.008.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. doi:10.1073/pnas.98.2.676.
Schwartz, S., Vuilleumier, P., Hutton, C., Maravita, A., Dolan, R. J., & Driver, J. (2005). Attentional load and sensory competition in human vision: modulation of fMRI responses by load at fixation during task-irrelevant stimulation in the peripheral visual field. Cerebral Cortex, 15(6), 770–786. doi:10.1093/cercor/bhh178.
Shirer, W. R., Ryali, S., Rykhlevskaia, E., Menon, V., & Greicius, M. D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex, 22(1), 158–165. doi:10.1093/Cercor/Bhr099.
Song, Y., Mu, K., Wang, J., Lin, F., Chen, Z., Yan, X., et al. (2014). Altered spontaneous brain activity in primary open angle glaucoma: a resting-state functional magnetic resonance imaging study. [Comparative Study Research Support, Non-U.S. Gov’t]. PLoS One, 9(2), e89493, doi:10.1371/journal.pone.0089493.
Tian, L., Ren, J., & Zang, Y. (2012). Regional homogeneity of resting state fMRI signals predicts stop signal task performance. NeuroImage, 60(1), 539–544. doi:10.1016/j.neuroimage.2011.11.098.
van de Ven, V. G., Formisano, E., Prvulovic, D., Roeder, C. H., & Linden, D. E. J. (2004). Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Human Brain Mapping, 22(3), 165–178. doi:10.1002/hbm.20022.
van den Heuvel, M. P., & Hulshoff Pol, H. E. (2010). Exploring the brain network: a review on resting-state fMRI functional connectivity. [review]. European Neuropsychopharmacology, 20(8), 519–534. doi:10.1016/j.euroneuro.2010.03.008.
Vidal, L., Ruiz, C., Villena, A., Diaz, F., & Perez de Vargas, I. (2004). Quantitative age-related changes in dorsal lateral geniculate nucleus relay neurons of the rat. Neuroscience Research, 48(4), 387–396. doi:10.1016/j.neures.2003.12.004.
von dem Hagen, E. A. H., Stoyanova, R. S., Baron-Cohen, S., & Calder, A. J. (2012). Reduced functional connectivity within and between ‘social’resting state networks in autism spectrum conditions. Social Cognitive and Affective Neuroscience, 8(6), 694–701. doi:10.1093/scan/nss053.
Wang, T. Y., Li, Q., Guo, M. X., Peng, Y. M., Li, Q. J., Qin, W., et al. (2014). Abnormal functional connectivity density in children with anisometropic amblyopia at resting-state. Brain Research, 1563, 41–51. doi:10.1016/J.Brainres.2014.03.015.
Wang, J. Q., Li, T., Sabel, B. A., Chen, Z. Q., Wen, H. W., Li, J. H., et al. (2016a). Structural brain alterations in primary open angle glaucoma: a 3 T MRI study. Scientific Reports, 6. doi:10.1038/Srep18969.
Wang, J., Li, T., Wang, N., Xian, J., & He, H. (2016b). Graph theoretical analysis reveals the reorganization of the brain network pattern in primary open angle glaucoma patients. European Radiology. doi:10.1007/s00330-016-4221-x.
Williams, A. L., Lackey, J., Wizov, S. S., Chia, T. M., Gatla, S., Moster, M. L., et al. (2013). Evidence for widespread structural brain changes in glaucoma: a preliminary voxel-based MRI study. [research support, non-U.S. Gov’t]. Investigative Ophthalmology & Visual Science, 54(8), 5880–5887. doi:10.1167/iovs.13-11776.
Yan, C. G., & Zang, Y. F. (2010). DPARSF: a MATLAB toolbox for "pipeline" data analysis of resting-state fMRI. Frontiers in Systems Neuroscience, 4. doi:10.3389/fnsys.2010.00013.
Yu, L., Xie, B., Yin, X., Liang, M., Evans, A. C., Wang, J., et al. (2013). Reduced cortical thickness in primary open-angle glaucoma and its relationship to the retinal nerve fiber layer thickness. [Research Support, Non-U.S. Gov’t]. PLoS One, 8(9), e73208, doi:10.1371/journal.pone.0073208.
Zeki, S., & Shipp, S. (1988). The functional logic of cortical connections. Nature, 335(6188), 311–317. doi:10.1038/335311a0.
Acknowledgments
This work was supported by National Natural Science Foundation of China (61271151, 91520202, and 81571649) and Youth Innovation Promotion Association CAS.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Author Jieqiong Wang, Author Ting Li, Author Peng Zhou, Author Ningli Wang, Author Junfang Xian, Author Huiguang He declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
Electronic supplementary material
ESM 1 Table S1
(DOCX 18 kb)
ESM 2 Table S2
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Li, T., Zhou, P. et al. Altered functional connectivity within and between the default model network and the visual network in primary open-angle glaucoma: a resting-state fMRI study. Brain Imaging and Behavior 11, 1154–1163 (2017). https://doi.org/10.1007/s11682-016-9597-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-016-9597-3