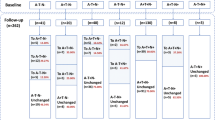
Abstract
Alzheimer’s disease (AD) is associated with a cascade of pathological events involving formation of amyloid-based neuritic plaques and tau-based neurofibrillary tangles, changes in brain structure and function, and eventually, cognitive impairment and functional disability. The precise sequence of when each of these disease markers becomes abnormal is not yet clearly understood. The present study systematically tested the relationship between classes of biomarkers according to a proposed model of temporal sequence by Jack et al. (Lancet Neurology 9:119–128, 2010). We examined temporal relations among four classes of biomarkers: CSF Aβ, CSF tau, neuroimaging variables (hippocampal volume, ventricular volume, FDG PET), and cognitive variables (memory and executive function). Random effects modeling of longitudinal data obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) was used to test hypotheses that putative earlier markers of AD predicted change in later markers, and that intervening markers reduced effects of earlier on later markers. Specifically, we hypothesized that CSF tau would explain CSF Aβ’s relation to neuroimaging and cognitive variables, and neuroimaging variables would explain tau’s relation to cognitive variables. Consistent with hypotheses, results indicated that CSF Aβ effects on cognition change were substantially attenuated by CSF tau and measures of brain structure and function, and CSF tau effects on cognitive change were attenuated by neuroimaging variables. Contrary to hypotheses, CSF Aβ and CSF tau were observed to have independent effects on neuroimaging and CSF tau had a direct effect on baseline cognition independent of brain structure and function. These results have implications for clarifying the temporal sequence of AD changes and corresponding biomarkers.




Similar content being viewed by others
References
Alzheimer’s Association. (2009). 2009 Alzheimer’s Disease facts and figures. Alzheimer’s and
Blom, G. (1958). Statistical estimates and transformed beta variables. New York: John Wiley & Sons, Inc.
Braak, H., Braak, E., & Bohl, J. (1993). Staging of Alzheimer-related cortical destruction. European Neurology, 33, 403–408.
Buerger, K., Ewers, M., Pirttila, T., et al. (2006). CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain, 129, 3035–3041.
Butterfield, D. A., Castegna, A., Lauderback, C. M., & Drake, J. (2002). Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiology of Aging, 23, 655–664.
Cole, D. A., & Maxwell, S. E. (2003). Testing mediational models with longitudinal data: questions and tips in the use of structural equation modeling. Journal of Abnormal Psychology, 112(4), 558–577.
Dale, A. M., Fischl, B., & Sereno, M. I. (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194.
De Leon, M. J., Convit, A., Wolf, O. T., Tarshish, C. Y., DeSanti, S., Rusinek, H., Tsui, W., Kandil, E., Scherer, A. J., Roche, A., Imossi, A., Thorn, E., Bobinski, M., Caraos, C., Lesbre, P., Schlyer, D., Poirier, J., Reisberg, B., & Fowler, J. (2001). Prediction of cognitive decline in normal elderly subjects with 2-[18F]fluoro-2-deoxy-d-glucose/positron-emission tomography (FDG/PET). PNAS, 98, 10966–10971.
Diggle, P. J., Heagerty, P., Liang, K. Y., & Zeger, S. L. (2002). The analysis of longitudinal data (2nd ed.). Oxford: Oxford University Press.
Dowling, N.M., Tomazewski Farias, S., Reed, B.R., Sonnen, J.A., Strauss, M.E., Schneider, J.A., Bennett, D.A., & Mungas, D. (2010). Neuropathological associates of multiple cognitive functions in two community-based cohorts of older adults. JINS. Epub ahead of print.
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355.
Fischl, B., Salat, D. H., van der Kouwe, A. J., Makris, N., Segonne, F., Quinn, B. T., et al. (2004). Sequence-independent segmentation of magnetic resonance images. NeuroImage, 23(Suppl 1), S69–S84.
Fischl, B., Sereno, M. I., & Dale, A. M. (1999). Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage, 9(2), 195–207.
Fischl, B., van der Kouwe, A., Destrieux, C., Halgren, E., Segonne, F., Salat, D. H., et al. (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22.
Habeck, C., Fostern, N. L., Pernecsky, R., Kurz, A., Alexopoulos, P., Koeppe, R. A., & Stern, Y. (2008). Multivariate and univariate neuroimaging biomarkers of Alzheimer’s disease. NeuroImage, 40(4), 1503–1515.
Hampel, H., Goernitz, A., & Buerger, K. (2003). Advances in the development of biomarkers for Alzheimer’s disease: from CSF total tau and AB1-42 proteins to phosphorylated tau protein. Brain Research Bulletin, 61, 243–253.
Hebert, L. E., Scherr, P. A., Bienias, J. L., Bennett, D. A., & Evans, D. A. (2003). Alzheimer disease in the U.S. population: prevalence estimates using the 2000 census. Archives of Neurology, 60(8), 1119–1122.
Howieson, D. B., Carlson, N. E., Moore, M. M., Wasserman, D., Abendroth, C. D., Payne-Murphy, J., & Kaye, J. A. (2008). Trajectory of mild cognitive impairment onset. Journal of the International Neuropsychological Society, 14(2), 192–198.
Ivnik, R. J., Malec, J. F., Tangalos, E. G., Petersen, R. C., Kokmen, E., & Kurland, L. T. (1990). The Auditory-Verbal Learning Test (AVLT): norms for ages 55 years and older. Psychological Assessment: A Journal of Consulting and Clinical Psychology, 2(3), 304–312.
Jack, C. R., Knopman, D. S., Jagust, W. J., Shaw, L. M., Aisen, P. S., Weiner, M. W., Petersen, R. C., & Trojanowski, J. Q. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology, 9, 119–128.
Jack, C. R., Shiung, M. M., Gunter, J. L., et al. (2004). Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology, 62, 591–600.
Jacqmin-Gadda, H., Commenges, D., & Dartigues, J. F. (2006). Random change point model for joint modeling of cognitive decline and dementia. Biometrics, 62(1), 254–260.
Jagust, W. J., Bandy, D., Chen, K., Foster, N. L., Landau, S. M., Mathis, C. A., Price, J. C., Reiman, E. M., Skovronsky, D., Koeppe, R. A., & Investigators, A. D. N. I. (2010). The ADNI PET Core. Alzheimer’s & Dementia, 6, 221–229.
Johnson, D. K., Storandt, M., Morris, J. C., & Galvin, J. E. (2009). Longitudinal study of the transition from healthy aging to Alzheimer disease. Archives of Neurology, 66(10), 1254–1259.
Kester, M. I., van der Vlies, A. E., Blankenstein, M. A., Pijnenburg, Y. A. L., van Elk, E. J., Scheltens, P., & van der Flier, W. M. (2009). CSF biomarkers predict rate of cognitive decline in Alzheimer’s disease. Neurology, 73, 1353–1358.
MacKinnon, D. P., Fairchild, A. J., & Fritz, M. S. (2007). Mediation analysis. Annual Review of Psychology, 58, 593–614.
Maxwell, S. E., & Cole, D. A. (2007). Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods, 12(1), 23–44.
Mungas, D., Beckett, L., Harvey, D., Tomaszewski Farias, S., Reed, B., Carmichael, O., & Decarli, C. (2010). Heterogeneity of cognitive trajectories in diverse older persons. Psychology and Aging, 25(3), 606–619.
Negash, S., Bennett, D. A., Wilson, R. S., Schneider, J. A., & Arnold, S. E. (2011). Cognition and neuropathology in aging: multidimensional perspectives from the rush religious orders study and rush memory and aging project. Current Alzheimer’s Research, 8, 336–340.
Oddo, S., Vasilevko, V., Caccamo, A., Kitazawa, M., Cribbs, D. H., & LaFerla, F. M. (2006). Reduction of the soluable Abeta and tau, but not soluable Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. Journal of Biological chemistry, 281, 39413–39423.
Petersen, R. C., Aisen, P. S., Beckett, L. A., Donohue, M. C., Gamst, A. C., Harvey, D. J., Jack, C. R., Jagust, W. J., Shaw, L. M., Toga, A. W., Trojanowski, J. Q., & Weiner, M. W. (2010). Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology, 74, 201–209.
Reiman, E. M., Chen, K., Alexander, G. E., Caselli, R. J., Bandy, D., Osborne, D., & Hardy, J. (2005). Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proceedings of the National Academy of Sciences of the United States of America, 102(23), 8299–8302.
Savva, G. M., Wharton, S. B., Ince, P. G., Forster, G., Matthews, F. E., & Brayne, C. (2009). Age, neuropathology, and dementia. The New England Journal of Medicine, 360, 2302–2309.
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., & Phelps, C. H. (2011). Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimer’s & Dementia, 7(3), 280–292.
Strozyk, D., Blennow, K., White, L. R., & Launer, L. J. (2003). CSF AB42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology, 60, 652–656.
Vemuri, P., Wiste, H. J., Weigand, S. D., Shaw, L. M., Trojanowski, J. Q., Weiner, M. W., Knopman, D. S., Petersen, R. C., Jack, C. R., & On behalf of the Alzheimer’s Disease Neuroimaging Initiative. (2009). MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology, 73, 287–293.
Verbeke, G., & Molenberghs, G. (2000). Linear mixed models for longitudinal data. New York: Springer.
Wilson, R. S., Beckett, L. A., Barnes, L. L., Schneider, J. A., Bach, J., Evans, D. A., & Bennett, D. A. (2002). Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging, 17(2), 179–193.
Yu, B., & Ghosh, P. (2010). Joint modeling for cognitive trajectory and risk of dementia in the presence of death. Biometrics, 66(1), 294–300.
Acknowledgments
This manuscript was a collaborative effort from the 2011 Friday Harbor Advanced Psychometrics Workshop (R13 AG030995), and used with gracious permission data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) downloaded on 6/1/11. Data collection and sharing for this project was funded by ADNI (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott; Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation.
Disclosures
There were no actual or potential conflicts of interest for any of the authors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.ucla.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Rights and permissions
About this article
Cite this article
Han, S.D., Gruhl, J., Beckett, L. et al. Beta amyloid, tau, neuroimaging, and cognition: sequence modeling of biomarkers for Alzheimer’s Disease. Brain Imaging and Behavior 6, 610–620 (2012). https://doi.org/10.1007/s11682-012-9177-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-012-9177-0