Abstract
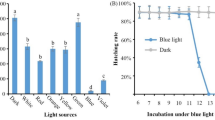
Bursaphelenchus xylophilus, causal agent of pine wilt disease, causes extensive damage worldwide. Strategies are needed to inhibit population growth or block the spread of the invasive nematode to control pine wilt disease. The gene daf-8 plays crucial roles in larval development and dauer formation in Caenorhabditis elegans, but little is known about its orthologue in B. xylophilus. In the present molecular characterization and functional analysis of daf-8 in B. xylophilus (Bx-daf-8), RT-qPCR showed that the expression of Bx-daf-8 gradually increased during the embryonic stage, peaked in the second-stage juvenile (J2), then dramatically dropped in the J3, and remained at that low level for the rest of its life. Bx-daf-8-transgenic C. elegans was employed to mimic the spatiotemporal expression of Bx-daf-8, which was expressed close to the pharynx during early embryogenesis and weakly throughout the whole body during late embryogenesis. It was observed in head neurons and tail ganglions throughout all postembryonic stages. B. xylophilus embryos were severely abnormal, and hatching rate decreased sharply after Bx-daf-8 knockdown. daf-16-1 and da-f16-2, downstream genes in the IIS pathway, also dropped sharply after Bx-daf-8 knockdown. We propose that TGFβ may crosstalk with the IIS pathway upstream of Bx-daf-16 and that daf-8 may act as a master regulator of daf-16 in B. xylophilus. However, knockdown of Bx-daf-8 did not lead to constitutive developmental arrest at the dauer larval stage, indicating that dauer entry in B. xylophilus might be controlled by several genes and is more complicated than in C. elegans. Bx-daf-8 alone did not control the dauer entry in B. xylophilus, but it was indispensable for embryogenesis, providing a potential target for suppressing population growth of B. xylophilus.





Similar content being viewed by others
Change history
11 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11676-022-01489-y
References
Burbea M, Lars D, Jeremy SD, Maria EG, Joshua MK (2002) Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in Caenorhabditis elegans. Neuron 35(1):107–120
Chen VB, Arendall WB, Jeffrey JH, Daniel AK, Robert MI, Gary JK, Laura WM, Jane SR, David CR (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr 66(1):12–21
Estevez M, Liliana A, Jeffrey LW, Patrice SA, Joan M, Donald LR (1993) The daf-4 gene encodes a bone morphogenetic protein receptor controlling Caenorhabditis elegans dauer larva development. Nature 365(6447):644–649
Futai K (2013) Pine wood nematode, Bursaphelenchus Xylophilus. Ann Rev Phytopathol 51(1):61–83
Georgi LL, Patrice SA, Donald LR (1990) daf-1, a Caenorhabditis elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell 61(4):635–645
Golden JW, Riddle LD (1984) A pheromone-induced developmental switch in Caenorhabditis elegans: temperature-sensitive mutants reveal a wild-type temperature-dependent process. Proc Natl Acad Sci USA 81(3):819–823
Inoue T, James HT (2000) Targets of TGF-β signaling in Caenorhabditis elegans dauer formation. Develop Biol 217(1):192–204
Kikuchi T, Cotton JA, Dalzell JJ, Hasegawa K, Kanzaki N, McVeigh P, Takanashi T, Tsai TJ, Assefa SA, Cock PJA (2011) Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. PLoS Pathog 7(9):e1002219
Kimble JE, White JG (1981) On the control of germ cell development in Caenorhabditis elegans. Develop Biol 81(2):208–219
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evolut 33(7):1870−1874.
Mamiya Y (1983) Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Ann Rev Phytopathol 21(4):201–220
Mamiya Y, Kiyohara T (1972) Description of Bursaphelenchus lignicolus n. sp. (Nematoda: Aphelenchoididae) from pine wood and histopathology of nematode-infested trees. Nematologica 18(1):120−124.
Massague J, Gomis RR (2006) The logic of TGFbeta signaling. FEBS Lett 580(12):2811–2820
McGehee AM, Benjamin JM, Peter J (2015) The DAF-7/TGF-β signaling pathway regulates abundance of the Caenorhabditis elegans glutamate receptor GLR-1. Mol Cell Neurosci 67:66–74
Narasimhan SD, Kelvin Y, Ankita B, Eun-Soo K, Srivatsan P, Heidi AT, Stuart KK (2011) PDP-1 links the TGF-β and IIS pathways to regulate longevity, development, and metabolism. PLoS Genet 7(4):e1001377
Nielsen H, Konstantinos DT, Søren B, Gunnar H (2019) A brief history of protein sorting prediction. Prot J 38(3):200–216
Okkema PG, Fire A (1994) The Caenorhabditis elegans NK-2 class homeoprotein CEH-22 is involved in combinatorial activation of gene expression in pharyngeal muscle. Development 120(8):2175–2186
Okkema PG, Eunju H, Christina H, Wei C, Andrew F (1997) The Caenorhabditis elegans NK-2 homeobox gene ceh-22 activates pharyngeal muscle gene expression in combination with pha-1 and is required for normal pharyngeal development. Development 124(20):3965–3973
Park D, Annette E, Donald LR (2010) Antagonistic Smad transcription factors control the dauer/non-dauer switch in Caenorhabditis elegans. Development 137(3):477–485
Park D, Karen LJ, Hyojin L, Terrance PS, Stefan T, Donald LR (2012) Repression of a potassium channel by nuclear hormone receptor and TGF-β signaling modulates insulin signaling in Caenorhabditis elegans. PLoS Genet 8(2):e1002519
Riddle DL, Margaret MS, Patrice SA (1981) Interacting genes in nematode dauer larva formation. Nature 290(5808):668–671
Savage-Dunn C, Lisa LM, Cole MZ, Andrew FR, Richard WP (2003) Genetic screen for small body size mutants in Caenorhabditis elegans reveals many TGFβ pathway components. Genesis 35(4):239–247
Schackwitz WS, Inoue T, Thomas JH (1996) Chemosensory neurons function in parallel to mediate a pheromone response in Caenorhabditis elegans. Neuron 17(4):719–728
Schilling SH, Michael BD, Xiao FW (2006) A phosphatase controls the fate of receptor-regulated Smads. Cell 125(5):838–840
Shaw WM, Luo S, Landis J, Ashraf J, Murphy CT (2007) The Caenorhabditis elegans TGF-beta Dauer pathway regulates longevity via insulin signaling. Curr Biol 17(19):1635–1645
Shi YG, Wang YF, Lata J, Yang HJ, Nikola PP (1998) Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-beta signaling. Cell 94(5):585–594
Tanaka SE, Mehmet D, Yasunobu M, Isheng JT, Ryusei T, Mark B, Yuko T, Kenji F, Natsumi K, Taisei K (2019) Stage-specific transcriptome of Bursaphelenchus xylophilus reveals temporal regulation of effector genes and roles of the dauer-like stages in the lifecycle. Sci Rep 9(1):e6080
Tang J, Ma RQ, Zhu NJ, Guo K, Guo YQ, Bai LQ, Yu HS, Hu JF, Zhang XY (2020) Bxy-fuca encoding alpha-L-fucosidase plays crucial roles in development and reproduction of the pathogenic pinewood nematode, Bursaphelenchus Xylophilus. Pest Manag Sci 76(1):205–214
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res 22:4673–4680
Tomlin KL, Clark SR, Ceri H (2004) Green and red fluorescent protein vectors for use in biofilm studies of the intrinsically resistant Burkholderia cepacia complex. J Microbiol Methods 57(1):95–106
Trent C, Tsuing N, Horvitz HR (1983) Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics 104(4):619–647
Vicente CLSL, Yoriko I, Ryoji S, Manuel M, Koichi H (2015) Catalases induction in high virulence pinewood nematode Bursaphelenchus xylophilus under hydrogen peroxide-induced stress. PLoS ONE 10(4):e0123839
Vowels JJ, Thomas JH (1992) Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics 130(1):105–123
Wu JW, Hu M, Chai J, Seoane J, Huse M, Li C, Rigotti DJ, Kyin S, Muir TW, Fairman R (2001) Crystal structure of a phosphorylated Smad2: recognition of phosphoserine by the MH2 domain and insights on Smad function in TGF-β signaling. Mol Cell 8(6):1277–1289
Wu YX, Wickham JD, Zhao LL, Sun JH (2019) CO2 drives the pine wood nematode off its insect vector. Curr Biol 29(13):619–620
Zhao LL, Zhang XX, Wei YN, Zhou J, Zhang W, Qin PJ, Chinta S, Kong XB, Liu YP, Yu HY, Hu SN, Zou Z, Butcher RA, Sun JH (2016) Ascarosides coordinate the dispersal of a plant-parasitic nematode with the metamorphosis of its vector beetle. Nat Commun 7:12341
Zhou LF, Chen FM, Xie LY, Pan HY, Ye JR (2017) Genetic diversity of pine-parasitic nematodes Bursaphelenchus xylophilus and Bursaphelenchus mucronatus in China. For Pathol 47(4):e12334
Zhou LF, Chen FM, Ye JR (2018) Selection of reliable reference genes for RT-qPCR analysis of Bursaphelenchus mucronatus gene expression from different habitats and developmental stages. Front Genet 9:e269
Zhou LF, Ji JJ, Zhu NJ, Guo K, Jia T, Bai LQ, Yu H, Hu JF (2021a) Molecular characterization and functional analysis of akt-1 in pinewood nematode, Bursaphelenchus xylophilus. Forest Pathol. https://doi.org/10.1111/efp.12647
Zhou LF, Ma XX, Zhu NJ, Zou QC, Guo K, Bai LQ, Yu HS, Hu JF (2021b) The role of mab-3 in spermatogenesis and ontogenesis of pinewood nematode, Bursaphelenchus Xylophilus. Pest Manag Sci 77(1):138–147
Zhu NJ, Bai LQ, Schütz S, Liu BJ, Liu ZY, Zhang XY, Yu HS (2016) Hu JF (2016) Observation and quantification of mating behavior in the pinewood nematode, Bursaphelenchus Xylophilus. J Visual Exp Jove 118:e54842
Author information
Authors and Affiliations
Contributions
Jinghan Wang and Huan Hong contributed equally to this study.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: The work was supported by the National Natural Science Foundation of China (31870633, 31700565, 31670652, 31570638 and 31270688) and National Key Research and Development Plan of China (2017YFD0600102-03).
The online version is available at http://www.springerlink.com
Corresponding editor: Tao Xu
In the Original Publication, the text “Acknowledgements sdfkjds" has been inadvertently appeared in the paper.
Rights and permissions
About this article
Cite this article
Wang, J., Hong, H., Xie, R. et al. Molecular characterization and functional analysis of daf-8 in the pinewood nematode, Bursaphelenchus xylophilus. J. For. Res. 33, 689–698 (2022). https://doi.org/10.1007/s11676-021-01335-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-021-01335-7