Abstract
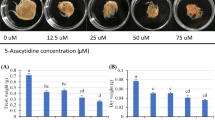
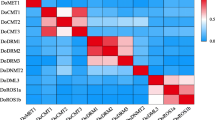
DNA methylation is widespread in plants and associated with plant development and defense mechanisms. However, the relationship between DNA methylation and plant secondary metabolism has rarely been reported. Here, when birch suspension cells were treated with 5-azacytidine (5-azaC), which blocks DNA methylation, triterpenoid accumulation was significantly promoted and antioxidant and defense enzymatic activity changed. For studying triterpenoid accumulation, 0.1 mM azaC was optimal. A qRT-PCR assay revealed increased expression of genes encoding key triterpenoid biosynthetic enzymes. Evaluation of methylation polymorphisms at CCGG sites showed that the methylation level was lower in cells treated with 5-azaC. These results demonstrated that 5-azaC treatment led to an increase in the production of triterpenoids in cell cultures through a mechanism that involved in DNA methylation, which resulted in the induction of genes encoding the key enzymes. The study provides evidence of a relationship between DNA methylation and regulation of secondary metabolism.







Similar content being viewed by others
References
Cheng CW, Chen LY, Chou CW, Liang JY (2015) Investigations of riboflavin photolysis via coloured light in the nitro blue tetrazolium assay for superoxide dismutase activity. J Photochem Photobiol, B 148:262–267
Dickerson DP, Pascholati SF, Hagerman AE, Butler LG, Nicholson RL (1984) Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol Plant Pathol 25(2):111–123
Dzubak P, Hajduch M, Vydra D, Hustova A, Kvasnica M, Biedermann D, Sarek J (2006) Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat Prod Rep 23(3):394–411
Eom HJ, Kang HR, Kim HK, Jung EB, Park HB, Kang KS, Kim KH (2016) Bioactivity-guided isolation of antioxidant triterpenoids from Betula platyphylla var. japonica bark. Bioorg Chem 66:97–101
Fan GZ, Zhang YG, Li K (2009) Preliminary optimization the extraction conditions of triterpene in callus of Betula platyphylla Suk. Chin Agric Sci Bull 25:55–58
Fieldes MA, Schaeffer SM, Krech MJ, Brown JC (2005) DNA hypomethylation in 5-azacytidine induced early-flwoering lines of flax. Theor Appl Genet 111:136–149
Gambino G, Perrone I, Gribaudo I (2008) A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem Anal 19(6):520–525
Guo Q, Liu Q, Smith AN, Liang G, Wang MB (2016) RNA Silencing in plants: mechanisms, technologies and applications in horticultural crops. Curr Genomics 17(6):476–489
Guo SL, Zhang DH, Wei HY, Zhao YN, Cao YB, Yu T, Wang Y, Yan XF (2017) Climatic factors shape the spatial distribution of concentrations of triterpenoids in barks of white birch (Betula Platyphylla Suk.) trees in northeast China. Forests 8(9):334
Hirasawa Y, Arai M, Imazeki F, Tada M, Mikata R, Fukai K, Miyazaki M, Ochiai T, Saisho H, Yokosuka O (2006) Methylation status of genes upregulated by demethylating agent 5-aza-2′-deoxycytidine in hepatocellular carcinoma. Oncology 71(1–2):77–85
Kim MY, Zilberman D (2014) DNA methylation as a system of plant genomic immunity. Trends Plant Sci 19(5):320–326
Kiselev KV, Dubrovina AS, Veselova MV, Bulgakov VP, Fedoreyev SA, Zhuravlev YN (2007) TherolBgene-induced overproduction of resveratrol in Vitis amurensis transformed cells. J Biotechnol 128:681–692
Kiselev KV, Tyunin AP, Manyakhin AY (2011) Resveratrol content and expression patterns of stilbene synthase genes in Vitis amurensis cells treated with 5-azacytidine. Plant Cell Tissue Organ Cult 105:65–72
Krasutsky PA (2006) Birch bark research and development. Nat Prod Rep 23(6):919–942
Lavania UC, Srivastava S, Lavania S, Basu S, Misra NK, Mukai Y (2012) Autopolyploidy differentially influences body size in plants, but facilitates enhanced accumulation of secondary metabolites, causing increased cytosine methylation. Plant J 71(4):539–549
Li P, Fang GG, Sun CZ (1995) Wood characteristics of pulpwood. Chem Indus For Prod 15(Suppl):13–18
Lomkova EA, Chytil P, Janoušková O, Mueller T, Lucas H, Filippov SK, Etrych T (2016) Biodegradable micellar HPMA-based polymer-drug conjugates with betulinic acid for passive tumor targeting. Biomacromol 17(11):3493–3507
Manai J, Kalai T, Gouia H, Corpas FJ (2014) Exogenous nitric oxide (NO) ameliorates salinity-induced oxidative stress in tomato (Solanum lycopersicum) plants. J Soil Sci Plant Nutr 14(2):433–446
Newman JD, Chappell J (1999) Isoprenoid biosynthsis in plants: carbon partitioning within the cytoplasmic pathway. Crit Rev Biochem Mol Biol 34:95–106
Petronelli A, Pannitteri G, Testa U (2009) Triterpenoids as new promising anticancer drugs. Anticancer Drugs 20(10):880–892
Schübeler D (2015) Function and information content of DNA methylation. Nature 517(7534):321–326
Shang GM, Wu JC, Yuan YJ (2004) Improved cell growth and Taxol production of suspension-cultured Taxus chinensis var. mairei in alternating and direct current magnetic fields. Biotech Lett 26:875–880
Singh J, Sabir F, Sangwan RS, Narnoliya LK, Saxena S, Sangwan NS (2015) Enhanced secondary metabolite production and pathway gene expression by leaf explants-induced direct root morphotypes are regulated by combination of growth regulators and culture conditions in Centella asiatica (L.) urban. Plant Growth Regul 75(1):55–66
Strauss J, Reyes-Dominguez Y (2011) Regulation of secondary metabolism by chromatin structure and epigenetic codes. Fungal Genet Biol 48(1):62–69
Tan ZY, Yuan HJ (2010) Determination of oleanolic acid in cortex Aralia elatae by HPLC. Chin J Inf Tradit Chin Med 17:46–47
Tyunin AP, Kiselev KV, Zhuravlev YN (2012) Effect of 5-azacytidine induced DNA demethylation on methyltransferase gene expression and resveratrol production in cell cultures of Vitis amurensis. Plant Cell Tiss Organ Cult 111:91–100
Tyunin AP, Kiselev KV, Karetin YA (2013) Differences in the methylation patterns of the VaSTS1 and VaSTS10 genes of Vitis amurensis Rupr. Biotech Lett 35(9):1525–1532
Vranová E, Coman D, Gruissem W (2013) Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu Rev Plant Biol 64:665–700
Wang YJ, Rong H, Xie T, Jiang JJ, Wu J, Wang Y (2016) Comparison of DNA methylation in the developing seeds of yellow-and black-seeded Brassica napus through MSAP analysis. Euphytica 209(1):157–169
Xiong LZ, Xu CG, Saghai Maroof MA, Zhang Q (1999) Patterns of cytosine methyaltion in an elite rice hybrid and its parental lines, detected by a methylation-sensitive amplification polymorphism technique. Mol Genet Genomics 261:439–446
Yadav DK, Kalani K, Singh AK, Khan F, Srivastava SK, Pant AB (2014) Design, synthesis and in vitro evaluation of 18 β-glycyrrhetinic acid derivatives for anticancer activity against human breast cancer cell line MCF-7. Curr Med Chem 9:1160–1170
Yang KL, Zhuang ZH, Zhang F, Song FQ, Zhong H, Ran FL, Yu S, Xu GP, Lan FX, Wang SH (2015) Inhibition of aflatoxin metabolism and growth of Aspergillus flavus in liquid culture by a DNA methylation inhibitor. Food Addit Contam: A 32(4):554–563
Zeng FS, Qian JJ, Luo W, ZhanYG Xin Y, Yang CP (2010) Stability of transgenes in long-term micropropagation of plants of transgenic birch (Betula platyphylla). Biotech Lett 32:151–156
Zeng FS, Liu K, Li SD, Zhan YG (2015) Crosstalk among nitric oxide, calcium and reactive oxygen species during triterpenoid biosynthesis in Betula platyphylla. Funct Plant Biol 42(7):643–654
Zhang H, Shibuya M, Yokota S, Ebizuka Y (2003) Oxidosqualene cyclases from cell suspension cultures of Betula platyphyllavar. japonica: molecular evolution of oxidosqualene cyclases in higher plants. Biol Pharm Bull 26:642–650
Zhang PY, Wang JG, Geng YP, Dai JR, Zhong Y, Chen ZZ, Zhu K, Wang XZ, Chen SY (2015) MSAP-based analysis of DNA methylation diversity in tobacco exposed to different environments and at different development phases. Biochem Syst Ecol 62:249–260
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Tao Xu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: The work was supported by The Fundamental Research Funds for the Central Universities (2572017EA05) and the National Natural Science Foundation of China (31870588).
The online version is available at http://www.springerlink.com.
Rights and permissions
About this article
Cite this article
Zeng, F., Li, X., Qie, R. et al. Triterpenoid content and expression of triterpenoid biosynthetic genes in birch (Betula platyphylla Suk) treated with 5-azacytidine. J. For. Res. 31, 1843–1850 (2020). https://doi.org/10.1007/s11676-019-00966-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-019-00966-1