Abstract
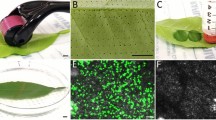
A method for transient gene expression was developed for western white pine (WWP, Pinus monticola Dougl. ex D.Don) using reporter gene uidA encoding β-glucuronidase (GUS). GUS was transiently expressed in cross sections of primary and secondary needles, cotyledons, and current and second year stems of WWP via vacuum-infiltration with Agrobacterium tumefaciens. Histochemical assays of cross sections of secondary needles showed stronger blue color indicating GUS expression at day 1 and 2 than on other days post agroinfiltration (dpa). GUS activity expressed inside WWP cells was confirmed using light microscopy. In fluorometric assays, GUS expression was high at 1 dpa and lasted until 4 dpa in detached secondary needles, while similarly high expression levels only lasted until 2 dpa in attached secondary needles then dropped significantly. Although the length of GUS-staining zones varied among different WWP organs and between growth and dormant seasons, all tested WWP tissues using the protocol had high levels of transient GUS expression. Thus, heterologous candidate genes or endogenous silencing can be expressed in various WWP tissues or organs using this agroinfiltration approach. The current protocol for efficient transient gene expression will aid functional genomics study of WWP and its pathogens and related conifer species.






Similar content being viewed by others
Abbreviations
- GUS:
-
β-glucuronidase
- MU:
-
4-methylumbelliferone
- MUG:
-
4-methylumbelliferyl-β-d-glucuronide
- WWP:
-
Western white pine
References
Andrieu A, Breitler JC, Siré C, Meynard D, Gantet P, Guiderdoni E (2012) An in planta, Agrobacterium-mediated transient gene expression method for inducing gene silencing in rice (Oryza sativa L.) leaves. Rice 5:23
Basu C, Kausch AP, Luo H, Chandlee JM (2003) Promoter analysis in transient assays using a GUS reporter gene construct in creeping bentgrass (Agrostis palustris). J Plant Physiol 160:1233–1239
Bhattacharya A, Sood P, Citovsky V (2010) The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection: phenolics in agrobacterium and rhizobium infection. Mol Plant Pathol 11(5):705–719
Błażejewska K, Kapusta M, Zielińska E, Tukaj Z, Chincinska IA (2017) Mature luffa leaves (Luffa cylindrica L.) as a tool for gene expression analysis by Agroinfiltration. Front Plant Sci 8:228
Blazquez M (2007) Quantitative GUS activity assay of plant extracts. Cold Spring Harb Protoc 2007. https://doi.org/10.1101/pdb.prot4690
Canevascini S, Caderas D, Mandel T, Fleming AJ, Dupuis I, Kuhlemeier C (1996) Tissue-specific expression and promoter analysis of the tobacco Itp1 gene. Plant Physiol 112:513–524
Christou P (1996) Transformation technology. Trends Plant Sci 1:423–431
Côté C, Rutledge RG (2003) An improved MUG fluorescent assay for the determination of GUS activity within transgenic tissue of woody plants. Plant Cell Rep 21(6):619–624
Du J, Rietman H, Vleeshouwers VGAA (2014) Agroinfiltration and PVX agroinfection in potato and Nicotiana benthamiana. J Vis Exp 83:e50971
Dunoyer P, Himber C, Voinnet O (2006) Induction, suppression and requirement of RNA silencing pathways in virulent Agrobacterium tumefaciens infections. Nat Genet 38:258–263
Evans T (ed) (2006) Transformation and microinjection. WormBook, ed. The C. elegans Research Community, WormBook. https://doi.org/10.1895/wormbook.1.108.1
Fang AF, Han YQ, Zhang N, Zhang M, Liu LJ, Li S, Lu F, Sun WX (2016) Identification and characterization of plant cell death-inducing secreted proteins from Ustilaginoidea virens. Mol Plant Microbe Interact 29:405–416
Fior S, Gerola PD (2009) Impact of ubiquitous inhibitors on the GUS gene reporter system: evidence from the model plants Arabidopsis, tobacco and rice and correction methods for quantitative assays of transgenic and endogenous GUS. Plant Methods 5:19
Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the ‘Gene-Jockeying’ tool. Microbiol Mol Biol Rev 67:16–37
Gelvin SB (2010) Plant proteins involved in Agrobacterium-mediated genetic transformation. Annu Rev Phytopathol 48:45–68
Gilissen LJ, Metz PL, Stiekema WJ, Nap JP (1998) Biosafety of E. coli beta-glucuronidase (GUS) in plants. Transgenic Res 7:157–163
Janssen BJ, Gardner RC (1990) Localized transient expression of GUS in leaf discs following cocultivation with Agrobacterium. Plant Mol Biol 14:61–72
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Johansen LK, Carrington JC (2001) Silencing on the spot. Induction and suppression of RNA silencing in the Agrobacterium-mediated transient expression system. Plant Physiol 126:930–938
Jones H, Ooms G, Jones MG (1989) Transient gene expression in electroporated Solanum protoplasts. Plant Mol Biol 13:503–511
Kapila J, De Rycke R, Van Montagu M, Angenon G (1997) An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci 122:101–108
Kim MJ, Baek K, Park CM (2009) Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis. Plant Cell Rep 28:1159–1167
Krenek P, Samajova O, Luptovciak I, Doskocilova A, Komis G, Samaj J (2015) Transient plant transformation mediated by Agrobacterium tumefaciens: principles, methods and applications. Biotechnol Adv 33:1024–1042
Krzymowska M, Konopka-Postupolska D, Sobczak M, Macioszek V, Ellis BE, Hennig J (2007) Infection of tobacco with different Pseudomonas syringae pathovars leads to distinct morphotypes of programmed cell death. Plant J 50:253–264
Lacroix B, Citovsky V (2013) The roles of bacterial and host plant factors in Agrobacterium-mediated genetic transformation. I J Dev Biol 57:467–481
Lee MW, Yang YN (2006) Transient expression assay by agroinfiltration of leaves. Methods Mol Biol 323:225–229
Levy M, Rachmilevitch S, Abel S (2005) Transient Agrobacterium-mediated gene expression in theArabidopsis hydroponics root system for subcellular localization studies. Plant Mol Biol Rep 23:179–184
Liu JJ, Ekramoddoullah AKM (2003) Root-specific expression of a western white pine PR10 gene is mediated by different promoter regions in transgenic tobacco. Plant Mol Biol 52:103–120
Liu ZH, Friesen TL (2012) Polyethylene glycol (PEG)-mediated transformation in filamentous fungal pathogens. In: Bolton MD, Thomma BPHJ (eds) Plant fungal pathogens. Humana Press, Totowa, pp 365–375
Liu JJ, Sturrock RN, Sniezko RA, Williams H, Benton R, Zamany A (2015) Transcriptome analysis of the white pine blister rust pathogen Cronartium ribicola: de novo assembly, expression profiling, and identification of candidate effectors. BMC Genom 16:678
Liu JJ, Sniezko RA, Zamany A, Williams H, Wang N, Kegley A, Savin DP, Chen H, Sturrock RN (2017) Saturated genic SNP mapping identified functional candidates and selection tools for the Pinus monticola Cr2 locus controlling resistance to white pine blister rust. Plant Biotechnol J 15:1149–1162
Lombardi R, Circelli P, Villani ME, Buriani G, Nardi L, Coppola V, Bianco L, Benvenuto E, Donini M, Marusic C (2009) High-level HIV-1 Nef transient expression in Nicotiana benthamiana using the P19 gene silencing suppressor protein of Artichoke Mottled Crinckle Virus. BMC Biotechnol 9:96
Mani T, Manjula S (2011) Optimization of Agrobacterium-mediated transient gene expression and endogenous gene silencing in Piper colubrinum Link. by vacuum infiltration. Plant Cell Tissue Organ Cult 105:113–119
Ohta S, Mita S, Hattori T, Nakamura K (1990) Construction and expression in tobacco of a β-Glucuronidase (GUS) reporter gene containing an intron within the coding sequence. Plant Cell Physiol 31(6):805–813
Panwar V, McCallum B, Bakkeren G (2013) Endogenous silencing of Puccinia triticina pathogenicity genes through in planta-expressed sequences leads to the suppression of rust diseases on wheat. Plant J 73:521–532
Shah KH, Almaghrabi B, Bohlmann H (2013) Comparison of expression vectors for transient expression of recombinant proteins in plants. Plant Mol Biol Rep 31:1529–1538
Takahashi T, Naito S, Komeda Y (1992) The Arabidopsis HSP18.2 promoter/GUS gene fusion in transgenic Arabidopsis plants: a powerful tool for the isolation of regulatory mutants of the heat-shock response. Plant J 2:751–761
Tang W (2001) Agrobacterium-mediated transformation and assessment of factors influencing transgene expression in loblolly pine (Pinus taeda L.). Cell Res 11:237–243
Thomas CE, Ehrhardt A, Kay MA (2003) Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet 4:346–358
Vitha S, Beneš K, Michalová M, Ondřej M (1993) Quantitative β-glucuronidase assay in transgenic plants. Biol Plant 35:151–155
Wu HY, Liu KH, Wang YC, Wu JF, Chiu WL, Chen CY, Wu SH, Sheen J, Lai EM (2014) AGROBEST: an efficient Agrobacterium-mediated transient expression method for versatile gene function analyses in Arabidopsis seedlings. Plant Methods 10:19
Xu KD, Huang XH, Wu MM, Wang Y, Chang YX, Liu K, Zhang J, Zhang Y, Zhang FL, Yi LM, Li TT, Wang RY, Tan GX, Li CW (2014) A rapid, highly efficient and economical method of Agrobacterium-mediated in planta transient transformation in living onion epidermis. PLoS ONE 9:e83556
Yang YN, Li RG, Qi M (2000) In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 22:543–551
Yasmin A, Debener T (2010) Transient gene expression in rose petals via Agrobacterium infiltration. Plant Cell Tissue Organ Cult 102:245–250
Acknowledgements
Authors appreciate Terry Holmes at CFS for help with microscopy and Gary Roke for providing WWP seedlings.
Author information
Authors and Affiliations
Contributions
The project was conceived by JJL. The experiments were designed and performed by ZM. Data were analyzed by ZM with the contribution of JJL, AZ, and HW. The manuscript was written by ZM and JJL with the contribution of AZ and HW.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: The work was partly supported by the CFS-GRDI fund.
The online version is available at http://www.springerlink.com
Corresponding editor: Tao Xu.
Rights and permissions
About this article
Cite this article
Ma, Z., Liu, JJ., Zamany, A. et al. Transient gene expression in western white pine using agroinfiltration. J. For. Res. 31, 1823–1832 (2020). https://doi.org/10.1007/s11676-019-00938-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-019-00938-5