Abstract
This study evaluated impacts of Coriaria nepalensis Wall. colonization on soil characteristics, vegetation structure and composition, regeneration status and expected future compositional changes, biomass and carbon stock in tree species of a mixed conifer forest of Central Himalaya. Three sites (1 ha each in an old landslide area) differing in Coriaria density (low: 20 individuals ha−1; medium: 120 indiv. ha−1; high: 190 indiv. ha−1) were used to enumerate the tree species. A total of 9 tree species and 2830 individuals were recorded from the three study sites that represented a gradient of Coriaria density. Number of species varied from 3 to 7 and the individuals from 690 to 1270 per site with lowest numbers at low Coriaria density and highest at medium Coriaria density. The number of seedlings increased with increasing Coriaria density, and the sites were unique in their seedling composition, indicating marked temporal dynamics. Site wise regeneration analysis showed that regeneration was poor at the site with low Coriaria density and good at the high-density site where many species emerged as seedlings. These results indicate that the ameliorative effects of Coriaria in terms of soil buildup, and accumulation of nutrients and organic matter helped more species to colonize the area. This facilitative ability of Coriaria can be used to restore degraded forest ecosystems of Indian Central Himalaya.
Similar content being viewed by others
Introduction
The biodiversity and population structure and dynamics of a plant community are strongly influenced by positive plant–plant interactions (plant facilitation). Many organisms modify, create or define habitats by altering the ecosystem and landscape properties (Berke 2010; Swamy et al. 2010; Hasui et al. 2017). These organisms have been formally defined as ecosystem engineers and represent a fundamental class of ecological interaction in most, if not all, ecosystems (Barker and Odling-Snee 2014; Kagan et al. 2016; Pearce 2011). Ecosystem engineers are a taxonomically diverse group, with representatives including vertebrates, invertebrates, algae, nonvascular plants and higher plants, especially woody species (Jones et al. 2006). In forest ecosystems, tree and shrubs can be considered physical ecosystem engineers since they modify their environment by patch formation that change the physical structures, the light regimes, the water flow and the redistribution of nutrients (Jones et al. 2006; Gosain et al. 2015; Naudiyal and Schmerbeck 2017; Mishra et al. 2017).
Coriaria nepalensis Wall. (Coriariaceae) is a common native, shrub species of the Central Himalayan region between 1200 and 2500 m a.s.l. Coriaria tends to be taller than the native understory shrubs and reaches a similar height or was shorter than the overstory trees. Accordingly, it fills a largely unexploited height class niche (small tree/large shrub). It is predominant in both disturbed and undisturbed forest ecosystems, often present in secondary forest and is a successful colonizer of landslide-affected or freshly exposed rocky and eroded slopes. It is a nitrogen-fixing actinorhizal plant that forms root nodules with actinomycete Frankia (Bargali and Bargali 2009). It can also facilitate growth of associate plants; therefore, it can change the composition and structure of the forest. Plant facilitation (the positive effect of one species on the establishment and growth of another plant species) is recognized as an important driving force in primary and secondary succession (Brooker 2006; Padilla and Pugnaire 2006). Facilitative or nurse plant effects are more evident in harsh, limiting environments where these plants ameliorate the abiotic conditions, thereby providing more suitable habitats for associated plant species (Wright et al. 2006). Clarkson and Clarkson (1995) documented the pivotal role of Coriaria arborea in the colonization of upper slopes of Mt Tarawera in New Zealand and argued that its presence is a necessary precursor for the establishment of forests.
In this study, we examined the impact of Coriaria nepalensis (hereafter, Coriaria) at three sites differing in Coriaria density in an old landslide area in a mixed conifer forest. The impact was measured in terms of species richness, individual density (tree, sapling and seedling), population structure, regeneration status, expected future change in composition, and the biomass and carbon stock of tree-layer vegetation. Our main objectives were to (1) determine the impacts of Coriaria colonization on vegetation structure and composition, (2) analyse the impact of Coriaria colonization on regeneration and expected change in composition of tree species, and (3) analyse the impact of Coriaria colonization on ecosystem functioning in a mixed conifer forest of Central Himalaya.
Materials and methods
Study area
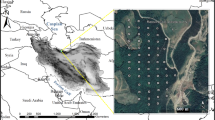
Ecologically, the Himalayan mountain chain can be divided into three different parts, i.e. Western Himalaya, Eastern Himalaya and Central Himalaya. The Central Himalaya include two divisions: the Kumaun Himalaya and the Garhwal Himalaya. The Kumaun Himalaya forms the northwestern part of the central Himalayan region between 28°44′–30°49′N latitudes and 78°45′–85°5′E longitudes. The study sites were selected between 1600–1850 m above mean sea level (29°36′56″–29°36′79″N, 79°46′03″–79°46′19″E) near Nainital town, Central Himalaya. Each site was further divided into three 1-ha subsites varying in Coriaria colonization in terms of density (low: 20 individuals ha−1; medium: 120 indiv. ha−1; high: 190 indiv. ha−1). The study area was dominated by conifers such as Pinus roxburghii and Cupressus torulosa (Fig. 1).
Climate
Nainital has long cold, often snowy winters and short summers with temperate and monsoon-type climate (Singh and Singh 1987) that is determined by the monsoon rhythms. The three main seasons are winter, which is usually cold and relatively dry (mid-December to mid-February), summer, which is warm and dry (April to mid-June), and a rainy period, which is warm and wet (mid-June to mid-September). The transition periods between summer and winter, and between winter and summer can be recognized as autumn (October–November) and spring (February–March), respectively. The rainy season accounts for about three-fourths of the annual rainfall (Fig. 2). The mean minimum temperature ranges from 2 °C (February) to 16.3 °C (July), and the mean maximum day temperature varies from 15.2 °C (January) to 26.8 °C (May).The annual rainfall of the area is 2320 mm, and the mean monthly rainfall ranges between 1.0 mm (November) and 763.0 mm (June). Generally, average humidity fluctuates near the saturation point during the monsoon and lowest during the summer season.
The study sites fall within the krol formation and Blaini formations (Valdiya 1980). The soil layer is thin due to the mainly sloping topography, and primarily acidic with a soil moisture of 14.1–27.4% and water holding capacity of 30.9–40.7%.
Experimental design and analysis
All trees and saplings in 10 randomly placed, 10 × 10 m quadrats at each site were measured for diameter at breast height (DBH, i.e., 1.37 m above the ground). Within each of these quadrats, 1 × 1 m subquadrats were used to assess seedlings. The vegetation data (DBH/Number of individual species) were quantitatively analyzed for abundance, density and frequency according to the formula given by Curtis and McIntosh (1950), A/F ratio and relative frequency, relative density and relative basal area (Phillips 1959). The relative values were summed to obtain the importance value index (IVI) of Curtis (1959). The diversity index (H′) was computed using the Shannon–Wiener information index (Shannon and Wiener 1963). The concentration of dominance (CD) was computed usingSimpson’s index (Simpson 1949). Index of similarity (IS) was calculated (Mueller-Dombois and Ellenberg 1974) as:
where A is the number of species at site A, B is the number of species at site B, and C is the common species in both the sites. Equitability (E) was calculated (Pielou 1966) as:
where H′ = Shannon–Wiener information index, S = number of species and In = natural log.
Population structure and regeneration
To analyze population structure and understand regeneration of different species, individuals were measured for diameter at breast height (DBH) with a measuring tape. At each site, all individuals were counted for each tree species. In addition to seedling and sapling classes (Shankar 2001), six or more classes based on DBH were arbitrarily established. The total numbers of individuals belonging to these size classes were calculated for each species at each site. The density of seedlings and saplings was considered as an indicator of the regeneration potential and the regeneration pattern of the study area.
The relative density (RD) of trees by size class was calculated as; total number of individuals in the size class for each species/total number of individuals in all size classes of the species) × 100.
Regeneration status of individual tree species was determined by the method of Shankar (2001) as good regeneration, seedlings > saplings > trees; fair regeneration, seedlings > saplings ≤ trees; poor regeneration, species survives only at sapling stage, not as seedlings (though there may be fewer, more or the same number of saplings compared with trees); no regeneration, seedlings and saplings are absent, only trees are present; new, no trees present, only seedlings and/or saplings.
Results and discussion
Colonization effects on soil characteristics
Soil gravel, bulk density and pH decreased in the order from the low to high density Coriaria colonization sites, while sand, silt and clay and porosity increased (Table 1). Because of its relationship to solubility and decomposition, pH is important in determining the availability of many elements and is a good indicator of forest fertility, with most macronutrients having maximum availability at pH 6.5–7.5 (Black 1968). Carbon and nitrogen increased significantly from the low to high Coriaria colonization sites (Table 1). Oakley et al. (2006) reported that in the Sierra Nevada, N-fixing Ceanothus species significantly enrich soils with available forms of N, the most limiting nutrient in many temperate forests.
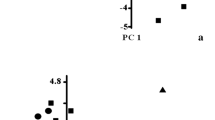
Principal component analysis (PCA) was done using scaling method 1 to investigate the interrelationship between soil quality indicators at the three sites (Fig. 3). In the PCA ordination plot that portrayed euclidean distances among the three sites, sites that are closer together have a small euclidean distance. PC1 and PC2 axes accounted for > 91% of the total variance; the remaining axes (PC3 to PC8) captured only about 8% of the total variance. Since more than half of the variance is captured in 2 dimensions (PC1 and PC2), the correspondence is “as good as it can be” because the 2 dimensions show as much of the variance as possible. Maximum loading on PC1 axis was obtained by the N component (0.612), while minimum loading was obtained by the sand component (0.753). Thus, maximum controlling was achieved by the sand component among physical characteristics and by the N component among the chemical characteristics.
Colonization effects on vegetation structure and composition
Total 56 species belonging to 35 families were reported across the entire study area. Of these, 10 were tree species, 15 were shrubs species, and 31 were herbs (Fig. 4). Pteridophytes were represented by 1 and gymnosperms by 2; remaining the 53 species were angiosperms. The richest families were Asteraceae with 8 species and Rosaceae with 7. Among 35 reported families from the study sites, 25 families were represented by one species. Tree species contributed 18%, shrubs contributed 27%, and herbs 55% of the total vegetation (Fig. 4). Reported species richness in the range of 30–40 was in accordance with the results of several related phytosociological investigations in the Himalaya (Kharkwal et al. 2009).
Number of species and number of individual varied from 4 to 8 and 690–1270 per site with former in site with low Coriaria colonization and later in site with medium Coriaria colonization (Table 2). Five species and 170 individual seedlings were reported at the site with high Coriaria colonization, while 7 species and 490 individual saplings were reported at the medium density site (Table 2). This result may be due to the facilitative effect of Coriaria, which have been reported to significantly enrich soils with available form of N (Bargali et al. 2003), the most limiting nutrient in temperate forests. When growing within or near plants that can fix nitrogen, seedlings and saplings may benefit from faster soil formation and enhanced soil fertility associated with N-fixing plants (Bargali et al. 2015). Four tree species were common to the three density sites, but present at different frequencies (Fig. 5). At the site with low Coriaria colonization, Pinus roxburghii was the most frequent while at the high Coriaria colonization site, Cupressus torulosa became more frequent.
The dominant and co-dominant species at the three life stages (i.e., tree, sapling and seedling) at different levels of Coriaria colonization are given in Table 3. Dominant and co-dominant species in each life stage varied from site to site, with no relationship between the adult and seedling life stage. The presence of few late successional species and higher number of early successional species indicates the successional nature of the community. Among shrub species, Pyracantha crenulata was dominant at the low and the medium Coriaria colonization sites, and Asparagus racemosus was dominant at the high Coriaria colonization site (Table 3). Rana et al. (2015) reported 8–11 species from a cypress forest in the Kumaun Himalaya. Species richness increased with increasing colonization, while density tended to fluctuate. The Coriaria density had a weak positive correlation with sapling density and tree basal area, while tree density and seedling density were positively correlated (Fig. 6).
Colonization effects on diversity indexes
Species diversity for trees increased from the low colonization (1.03) to high colonization (2.59) sites, while the concentration of dominance (CD) showed a reverse trend (Table 4). The values of equitability for trees were highest (1.25) at the high colonization site and lowest (0.75) at the low colonization site, indicating equal dominance by most species at the site with high Coriaria colonization. For saplings, the diversity ranged from 1.73 to 2.14, and concentration of dominance varied between 0.28 and 0.34. Equitability values of saplings were lowest (1.09) at the site with medium colonization and highest (1.25) at the site with low colonization. Diversity of seedlings ranged from 0.78 to 2.02, while the CD varied from 0.27 to 0.64. These values are within the range reported by Bargali et al. (2013) for an oak forest in the Central Himalaya. Equitability of seedlings was highest (1.12) at the medium colonization and lowest (1.44) at the low colonization site (Table 4). Species diversity for shrubs increased from the low colonization site (2.46) to the high colonization site (2.94). The values of equitability were highest (1.26) at the site with low Coriaria colonization and lowest (1.17) at the medium Coriaria colonization site. The higher species richness and diversity in the shrub layer than in the tree layer may be due to the formation of forest gaps, which provide an opportunity for the invasion of more shrubs. Whittaker (1972) also suggested that the dominance of one stratum affects the diversity of another stratum.
Colonization effects on present status of regeneration
The regeneration potential of low Coriaria colonization site showed that seedlings of only two species (C. nepalensis and Q. leocotrichophora) were present. These species can be referred to as fair reproducers because they reproduced well in the immediate past and continue to do so at the present. At a lower rate, P. roxburghii and C. torulosa had individuals in the intermediate class size while established seedling were absent, indicating that these species reproduce well at first but at present their regeneration has stopped (Fig. 7). Individuals of A. indica were only present as young trees (e.g., size class C), indicating its accidental presence.
The regeneration potential of the medium Coriaria colonization site does not seemed to be as good because only two seedling species were present (P. roxburghii and A. indica). L. umbrosa was represented by only an intermediate size class (C) larger than a sapling, thus, either present as relicts or nomads (Van Steenis 1958). A. indica had more individuals in lower size classes than in the higher size classes. According to Knight (1975), this structure represents frequent reproduction. For C. torulosa, Q. leucotrichophora, P. cerasoides, and P. pashia, the concentration of individuals in intermediate size classes was high with a general absence or negligible representation of individuals at higher and lower size classes (Fig. 7). According to West et al. (1981), such a population is on its way to extinction if such a trend continues, and Knight (1975), Bargali et al. (1987, 1989) referred to species with this type of population structure as an infrequent reproducer.
The regeneration potential of site with high Coriaria colonization was better because many species were represented in the seedling size class (C. nepalensis, L. umbrosa, A. indica, Q. leucotrichophora and S. cumini), indicating that the population has the potential for frequent reproduction (Knight 1975; Bargali et al. 2013; Kittur et al. 2014) and may be due to the capacity of nurse plants such as Coriaria, to alters the environment aboveground (canopy effect) and belowground (soil effect) and benefit seedling establishment (Lorena et al. 2005; Tsheboeng et al. 2017). For A. oblongum, more individuals were present in the intermediate size class with fewer in the lower and higher size classes (Fig. 7), a pattern of population structure that indicates infrequent reproduction (Knight 1975; Kittur et al. 2014). The presence of individuals in all size classes except seedlings for C. torulosa indicated that this species reproduced well at first but at present its regeneration has stopped. Across the site, regeneration was poor at the site with low Coriaria density and good at the site with high Coriaria density.
Colonization effects on expected compositional changes
Facilitation of seedlings by adult plants underlies successful regeneration of many species under stressful conditions, thereby playing a key role in shaping the structure of plant communities. In general, soil enrichment by nitrogen-fixing plants has been shown to have beneficial effects on neighbors in nutrient-poor environments (Shumway 2000). On the basis of the population structure of individual tree species at each site, we can draw the following conclusions:
-
At low Coriaria density, P. roxburghii and C. torulosa will remain dominant species, but later they may be replaced by Q. leucotrichophora.
-
At medium Coriaria density, the dominant species C. torulosa may later be replaced by A. indica, while P. roxburghii will be the dominant species.
-
At high Coriaria density, C. torulosa may remain dominant for some time but due to the absence of established seedlings, it may be replaced by Q. leucotrichophora and/or A. indica. Codominant species C. nepalensis will maintain its status, while associated species such as L. umbrosa and S. cumini will expand their population. The absence of seedlings and saplings of P. roxburghii will result in exclusion of this species from the site.
Dominance diversity curves
Dominance diversity curves (based on IVI for tree species and PV for shrub species) were drawn to interpret the community organization in terms of resource share and niche space (Fig. 8). The low and medium Coriaria colonization sites had one dominant species, while the high Coriaria colonization site had a relatively greater equitable share of resources among the various species.
Similarity index
On the basis of percentage similarity, low and medium colonization sites were 66.7% similar in the tree layer and 58.8% similar in the shrub layer (Table 5). The low and high colonization sites were 66.7% similar in the tree layer and 31.6% similar in the shrub layer. The medium and high colonization sites were 75% similar in the tree layer and 54.5% similar in the shrub layer. In total, the maximum similarity was recorded between low and medium (66.7%) and between medium and high colonization sites (66.7%), while low and high colonization sites had the lowest (51.8%) similarity.
Colonization effect on tree biomass and sequestered carbon stock
Using an allometric equation (Adhikari et al. 1998) relating biomass of different components with CBH and tree density, biomass was calculated for the different tree species at the study sites. At the low Coriaria colonization site, P. roxburghii contributed the most biomass (84.5 t ha−1) and A. indica had the lowest (7.6 t ha−1). The total biomass at this site was 150.6 t ha−1, of which 79.3% was aboveground and 18.8% belowground. Total sequestered carbon stock at the low Coriaria colonization site was 71.5 t ha−1and distributed similarly to biomass among components (Table 6).
At the medium Coriaria colonization site, C. torulosa had the most biomass (85.7 t ha−1) and Q. leucotrichophora had the least (4.0 t ha−1). The total biomass at this site was 234.4 t ha−1, of which 79.7% was aboveground and 20.6% belowground. Of the total biomass, boles contributed 79.0%, bole bark 6.9%, branches 21.2%, twigs 7.8%, foliage 5.9%, cones 0.03%, stump roots 12.5%, lateral roots 5.5% and fine roots 2.6% (Table 5). The total carbon stock in the tree layer pool was 111.44 t ha−1.
Among species at the high Coriaria colonization site, highest biomass was reported for Q. leucotrichophora (101.8 t ha−1) and lowest for S. cumini (5.0 t ha−1). The total biomass at the low Coriaria colonization site was 351.0 t ha−1, with 81.61% aboveground and 18.7% belowground. Among the tree components, 37.2% of the biomass was contributed by boles, 6.8% by bole bark, 21.3% by branches, 8.3% by twigs, 7.9% by foliage, 0.08% by cones, 13.9% by stump roots, 2.2% by lateral roots and 2.6% by fine roots. The total carbon storage at this site was 166.7 t ha−1.
Colonization effect on sapling layer biomass and sequestered carbon stock
The total biomass of the sapling layer at the low Coriaria colonization site was 32.7 t ha−1, with 77.6% aboveground, 22.5% belowground. Of the total biomass, 13.9% was contributed by boles, 28.2% by bole bark, 14.9% by branches, 11.2% by twigs, 9.3% by foliage, 11.6% by stump roots, 2.3% by lateral roots and 8.5% by fine roots. Among the species at this site, L. umbrosa contributed the most biomass (17.5 t ha−1), P. pashia the least (1.6 t ha−1). Total sequestered carbon stock in this layer was 15.5 t ha−1 (Table 7).
At the medium Coriaria colonization site, A. indica contributed the most biomass (32.7 t ha−1), C. nepalensis the least (0.8 t ha−1). The total biomass for the sapling layer at this site was 54.8 t ha−1; 57.3% was aboveground and 34.6% belowground. For the above ground components, boles contributed 26.63% biomass followed by branches (15.5%), twigs (8.8%), bole bark (8.7%), foliage (6.1%) and cone (0.3%). Biomass contribution in belowground component from highest to lowest was stump roots (18.1%) > lateral roots (11.7%) > fine roots (4.2%). Total sequestered carbon stock was 26.0 t ha−1.
At the high Coriaria colonization site, saplings of L. umbrosa contributed the most biomass (34.44 t ha−1) and C. torulosa the least (0.47 t ha−1). The total biomass was 52.53 t ha−1, with 79.25% aboveground and 20.80% belowground. Of the total biomass, boles contributed 11.09% biomass, bole bark 33.39%, branches 14.07%, twigs 11.68%, foliage 9.00%, stump roots7.41%, lateral roots 1.68% and fine roots11.73%. Total sequestered carbon stock was 24.95 t ha−1 (Table 7).
Impacts of Coriaria colonization on ecosystem functioning
Lambdon et al. (2008) reported that the ability of nitrogen-fixing plants to fix atmospheric nitrogen may significantly impact ecosystem functioning. In the present study, Coriaria colonization was associated with an increase in tree density, basal area, biomass and sequestered carbon stock (Fig. 5, Table 6). These results suggested that since total aboveground biomass tends to increase due to colonization by Coriaria, there is potential for increased ecosystem carbon storage. The increasing seedling density with the increase in Coriaria density indicates that species regeneration was positively affected by the presence of Coriaria, possibly due to altered soil conditions. Alteration to vegetation composition brought about by Coriaria colonization (Table 3) may have impact other ecosystem functions such as water and carbon cycling since species differ markedly in their functional traits and resource-use strategies (Werner et al. 2010). Furthermore, Bargali et al. (2003) documented that the moisture and nutrient status of soil was positively impacted by the presence of Coriaria. These results indicated that ecosystem vulnerability to drought and nutrient stress may be substantially reduced in colonized sites.
Colonization of the sites by Coriaria increased total soil nitrogen and impacted ecosystem nutrient cycling. With increasing density of Coriaria, the C:N ratio decreased, indicating that rates of potential nitrification increased with increasing density of Coriaria. Overall, evidence is increasing that colonization by the nitrogen-fixing Coriaria significantly modifies the native habitat to an extent that accelerates the restoration attempts (Joshi et al. 2001; Bargali et al. 2015).
Conclusions
Here we documented that C. nepalensis significantly changed vegetation structure and filled a nearly unexploited height class niche (small tree/large shrub) in a mixed conifer forest. Coriaria colonization increased tree density, basal area, biomass and diversity. In addition, it also increased the regeneration potential of tree species. Interestingly, the dominant tree species of the study sites (C. torulosa and P. roxburghii) failed to produce seedlings. Coriaria colonization made the site suitable for the growth of late successional species like Q. leucotrichophora and A. indica. Absence of trees in higher size classes for most of the species indicates the successional trend of the community. The presence of a sufficient number of seedlings on the site with high Coriaria colonization indicates better survival of species like L. umbrosa, S. cumini and A. indica. These effects, combined with pronounced differences in ecological strategy and high drought tolerance of the colonizer (Bargali and Tewari 2004), indicate that the colonized habitat may have decreased drought vulnerability. Furthermore, in terms of restoration prospects, the symbiotic nitrogen-fixing ability of Coriaria played a very important role in improving habitat quality as indicated by the better growth of associated plants, which will help to restore degraded hill slopes in the Central Himalaya.
References
Adhikari BS, Dhaila S, Rawat YS (1998) Structure of Himalayan moist temperate Cypress forest at and around Nainital, Kumaun Himalayas. Oecol Montana 7:21–31
Bargali K, Bargali SS (2009) Effect of phosphorus nutrition on growth and mycorrhizal dependency of Coriaria nepalensis seedlings. Nat Sci 7(6):19–24
Bargali K, Tewari A (2004) Growth and water relation parameters in drought stressed Coriaria nepalensis seedlings. J Arid Environ 58:505–512
Bargali SS, Tewari JC, Rawat YS, Singh SP (1987) Woody vegetation in high elevation blue—pine mixed oak forest of Kumaun Himalaya, India. In: Pangty YPS, Joshi SC (eds) Western Himalaya: environment, problems and development. Gyanodaya Parakashan, Nainital, pp 121–155
Bargali SS, Rana BS, Rikhari HC, Singh RP (1989) Population structure of Central Himalayan blue pine (Pinus wallichiana) forest. Environ Ecol 7:431–436
Bargali K, Joshi B, Goel D (2003) Impact on soil characteristic and understory vegetation of Coriaria nepalensis in natural landslide area. Ecoprint 10(1):43–46
Bargali K, Bisht P, Khan A, Rawat YS (2013) Diversity and regeneration status of tree species at Nainital Catchment, Uttarakhand, India. Int J Biodivers Conserv 5:270–280
Bargali K, Maurya NR, Bargali SS (2015) Effect of a nitrogen-fixing actinorhizal shrub on herbaceous vegetation in a mixed conifer forest of Central Himalaya. Curr World Environ 10(3):957–966
Barker G, Odling-Snee J (2014) Integrating ecology and evolution: niche construction and ecological engineering. In: Baker G, Deojardins E, Pearce T (eds) Entangled life. Springer, Dordrecht, pp 187–211
Berke SK (2010) Functional groups of ecosystem engineers: a proposed classification with comments on current issues. Integr Comp Biol 50:147–157
Black CA (1968) Soil plant relations. Wiley, New York
Brooker RW (2006) Plant–plant interactions and environmental change. New Phytol 171:271–289
Clarkson BR, Clarkson BD (1995) Recent vegetation changes on Mount Tarawera Rotorua, New Zealand. NZ J Bot 33:339–354
Curtis JT (1959) The vegetation of Wisconsin. University of Wisconsin Press, Madison
Curtis JT, McIntosh RP (1950) The interrelations of certain analytic and synthetic phytosociological characters. Ecology 31:434–455
Gosain BG, Negi GCS, Dhyani PP, Bargali SS, Saxena R (2015) Ecosystem services of forests: carbon stock in vegetation and soil components in a watershed of Kumaun Himalaya, India. Int J Ecol Environ Sci 41(3–4):177–188
Hasui E, Silva VX, Cunha RGT, Ramos FN, Ribeiro MC, Sacramento M, Coelho MTP, Pereira DGS, Ribeiro BR (2017) Additions of landscape metrics improve predictions of occurrence of species distribution models. J For Res 28(5):963–974
Jones CG, Cutierrez JL, Groffman PM, Shachak M (2006) Linking ecosystem engineers to soil processes: a framework using the Jenny State Factor Equation. Eur J Soil Biol 42(supplement 1):S39–S53
Joshi B, Singh SP, Rawat YS, Goel D (2001) Facilitative effect of Coriaria nepalensis on species diversity and growth of herbs on severely eroded hill slopes. Curr Sci 80(5):678–682
Kagan TP, Zaady E, Shachak M, Karnieli A (2016) Transformation of shrub lands to forests: the role of woody species as ecosystem engineers and landscape modulators. For Ecol Manag 361:257–268
Kharkwal G, Rawat YS, Pangtey YS (2009) An ordination of the forest communities in Nainital catchment of Kumaun Himalaya. J Environ Biol 30(5):853–857
Kittur B, Swamy SL, Bargali SS, Jhariya MK (2014) Wildland fires and moist deciduous forests of Chhattisgarh, India: divergent component assessment. J For Res 25(4):857–866
Knight DH (1975) A phytosociological analysis of species-rich tropical forest on Barro Colorado Island, Panama. Ecol Monogr 45:259–284
Lambdon PW, Pyšek P, Basnou C, Hejda M, Arianoutsou M, Essl F, Jarošík V, Pergl J, Winter M, Anastasiu P, Andriopoulos P, Bazos I, Brundu G, Celesti-Grapow L, Chassot P, Delipetrou P, Josefsson M, Kark S, Klotz S, Kokkoris Y, Kühn I, Marchante H, Perglová I, Pino J, Vilá M, Zikos A, Hulme PE (2008) Alien flora of Europe: species diversity, temporal trends, geographical patterns and research need. Preslia 80:101–149
Lorena GA, Gomez JM, Zamora R, Boettinger JL (2005) Canopy vs soil effects of shrubs facilitating tree seedlings in Mediterranean montane ecosystems. J Veg Sci 16:191–198
Mishra G, Das PK, Borah R, Dutta A (2017) Investigation of phytosociological parameters and physico-chemical properties of soil in tropical semi- evergreen forests of Eastern Himalaya. J For Res 28(3):513–520
Naudiyal N, Schmerbeck J (2017) The changing Himalayan landscape: pine-oak forest dynamics and the supply of ecosystem services. J For Res 28(3):431–443
Oakley BB, North MP, Franklin JF (2006) Facilitative and competitive effects of a N-fixing shrubs on white fir saplings. For Ecol Manag 233:100–107
Padilla FM, Pugnaire FI (2006) The role of nurse plants in the restoration of degraded environments. Front Ecol Environ 4:196–202
Pearce T (2011) Ecosystem engineering, experiment and evolution. Biol Philos 26:793–812
Phillips EA (1959) Method of vegetation study. Holt Rinehart and Winston, Inc., New York
Pielou EC (1966) Species diversity and pattern diversity in the study of ecological succession. J Theor Biol 10:370–383
Rana S, Bargali K, Bargali SS (2015) Assessment of plant diversity, regeneration status, biomass and carbon stock in a Central Himalayan cypress forest. Int J Biodivers Conserv 7(6):321–329
Shankar Uma (2001) A case of high tree diversity in a Sal (Shorea robusta) dominated lowland forest of Eastern Himalaya: floristic composition, regeneration and conservation. Curr Sci 81:776–786
Shannon CE, Weiner W (1963) The mathematical theory of communication. University of Illinois Press, Urbana
Shumway SW (2000) Facilitative effects of a sand dune shrub on species growing beneath the shrub canopy. Oecologia 124:138–148
Simpson EH (1949) Measurement of diversity. Nature 163:688
Singh JS, Singh SP (1987) Forest vegetation of Himalaya. Bot Rev 53:80–192
Swamy SL, Dutt CBS, Murthy MSR, Mishra A, Bargali SS (2010) Floristics and dry matter dynamics of tropical wet evergreen forests of Western Ghats, India. Curr Sci 99(3):353–364
Tsheboeng G, Murray-Hudson M, Kashe K (2017) Regeneration status of riparian tree species in two sites that differ in land use in the Oakvango delta, Botswana. J For Res 28(5):1073–1082
Valdiya KS (1980) Geology of Kumaun Lesser Himalaya. Wadia Institute of Himalayan Geology, Dehradun
Van Steenis CGGJ (1958) Rejuvenation as a factor for judging the status of vegetation types. The biological nomad theory. In: Proceedings of the Kandy symposium on study of tropical vegetation. UNESCO, Paris, pp 159–163
Werner C, Zumkier U, Beyschleg W, Maguas C (2010) High competitiveness of a resource demanding Acacia under low resource supply. Plant Ecol 206:83–96
West DC, Shugart JHH, Ranney JW (1981) Population structure of forest over a large area. For Sci 27:701–710
Whittaker RH (1972) Evolution and measurement of species diversity. Taxon 21:213–251
Wright JP, Jones CG, Boeken B, Shachak M (2006) Predictability of ecosystem engineering effects on species richness across environmental variability and spatial scales. J Ecol 94:815–824
Mueller-Dombois D, Ellenburg H (1974) Aims and methods of vegetation ecology. Wiley, Hoboken
Author information
Authors and Affiliations
Corresponding author
Additional information
Project funding: The work was partially supported by a project grant from University Grant Commission, New Delhi.
The online version is available at http://www.springerlink.com
Corresponding editor: Tao Xu.
Rights and permissions
About this article
Cite this article
Mourya, N.R., Bargali, K. & Bargali, S.S. Impacts of Coriaria nepalensis colonization on vegetation structure and regeneration dynamics in a mixed conifer forest of Indian Central Himalaya. J. For. Res. 30, 305–317 (2019). https://doi.org/10.1007/s11676-018-0613-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-018-0613-x