Abstract
Objective
Impaired signal transduction is associated with tumorigenesis and progression of various kinds of human cancers. Transforming growth factor (TGF)-beta/Smad and ras-mitogen activated protein kinase (MAPK) are two major signal transduction pathways for adjusting cell proliferation and differentiation. Little is known about TGF-beta/Smad4 in non-small cell lung cancer (NSCLC). Hereby, we investigated the expression of Smad4 in NSCLC, its correlation with MAPK proteins (including p38, ERK1 and JNK1 proteins) and their clinical significance in NSCLC.
Methods
The expressions of Smad4, p38, ERK1 and JNK1 were detected at protein level with Western blotting and immunohistochemistry, at transcription level with RT-PCR. Statistical analysis was performed for the comparisons of expressions of Smad4, p38, ERK1 and JNK1, and their correlation with various clinicopathological parameters and the prognosis of NSCLC.
Results
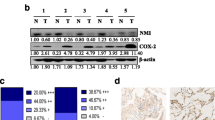
The levels of protein and mRNA expression of Smad4 in lung cancer tissues were significantly lower than in normal tissues (P<0.05). All these four proteins were associated with TNM staging. There was a strongly negative correlation between p38 and Smad4. Expressions of Smad4, p38 and JNK1, as well as tumor differentiation and staging were significantly correlated with the prognosis of NSCLC by univariate analysis. By multivariate analysis, only Smad4, p38, tumor differentiation and staging were correlated with the prognosis. Taken together, the negative expression of p38 and positive expression of Smad4 were associated with a better prognosis of NSCLC.
Conclusion
Smad4 could be of vital importance for the initiation and development of NSCLC. The expression of Smad4 might be inhibited by p38, supporting a cross-talk between main proteins of TGF-beta/Smad and ras-MAPK signal transduction pathways. Smad4 and p38 could be possible prognostic factors for NSCLC.
Similar content being viewed by others
References
Attisano L, Wrana JL. Signal transduction by members of the transforming growth factor-beta superfamily[J]. Cytokine Growth Factor Rev 1996; 7: 327–339.
Mourstakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction[J]. J Cell Sci 2001; 114: 4359–4369.
Rubin GM. Signal transduction and the fate of the R7 photoreceptor in Drosophila[J]. Trends Genet 1991; 7: 372–377.
Nishida E, Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways[J]. Trends Biochem Sci 1993; 18: 128–131.
Johnson GL, Lapadat R. Mitogen-activated protein ki8nase pathways mediated by ERK, JNK, and p38 protein kinases[J]. Science 2002; 298: 1911–1912.
Dumont N, Bakin AV, Arteaga CL. Autocrine transforming growth factor-beta signaling mediates Smad-independent motility in human cancer cells[J]. J Biol Chem 2003; 278: 3275–3285.
Xiao L, Lang W. A dominant role for the c-Jun NH2-terminal kinase in oncogenic ras-induced morphologic transformation of human lung carcinoma cells[J]. Cancer Res 2000; 60: 400–408.
Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription[J]. Nature 1998; 394: 909–913.
Watanabe H, de Caestecker MP, Yamada Y. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic ATDC5 cells[J]. J Biol Chem 2001; 276: 14466–14473.
Wrana JL. TGF-beta receptors and signaling mechanisms[J]. Miner Electrolyte Metab 1998; 24: 120–130.
Chiao PJ, Hunt KK, Grau AM, et al. Tumor suppressor gene Smad4/DPC4, its downstream target genes, and regulation of cell cycle[J]. Ann N Y Acad Sci 1999; 880: 31–37.
Xie W, Rimm DL, Lin Y, et al. Loss of Smad signaling in human colorectal cancer is associated with advanced disease and poor prognosis[J]. Cancer J 2003; 9: 302–312.
Maliekal TT, Antonym ML, Nair A, et al. Loss of expression, and mutations of Smad2 and Smad4 in human cervical cancer[J]. Oncogene 2003; 22: 4889–4897.
de Winter JP, Roelen BA, ten Dijke P, et al. DPC4 (SMAD4) mediates transforming growth factor-beta1 (TGF-beta1) induced growth inhibition and transcriptional response in breast tumor cells[J]. Oncogene 1997; 14: 1891–1899.
Campbell SL, Khosravi-Far R, Rossman KL, et al. Increasing complexity of Ras signaling[J]. Oncogene 1998; 17: 1395–1413.
Greenberg AK, Basu S, Hu J, et al. Selective p38 activation in human non-small cell lung cancer[J]. Am J Respir Cell Mol Biol 2002; 26: 558–564.
Yamagata H, Matsuzaki K, Mori S, et al. Acceleration of Smad2 and Smad3 phosphory-lation via-c-Jun NH(2)-terminal kinase during human colorectal caqrcinogenesis[J]. Cancer Res 2005; 65: 157–165.
Selvamurugan N, Kwok S, Alliston T, et al. Transforming growth factor-beta 1 regulation of collagenase-3 expression in osteoblastic cells by cross-talk between the Smad and MAPK signaling pathways and their components, Smad2 and Runx2[J]. J Biol Chem 2004; 279: 19327–19334.
Denton CP, Zheng B, Evans LA, et al. Fibroblast-specific expression of a kinase-deficient type II transforming growth factor beta(TGF-beta) receptor leads to paradoxical activation of TGF-beta signaling pathways with fibrosis in transgenic mice[J]. J Biol Chem 2003; 278: 25109–25019.
Jono H, Xu H, Kai H, et al. Transforming growth factor-beta-Smad signaling pathway negatively regulates nontypeable Haemophilus influenzae-induced MUC5AC mucin transcription via mitogen-activated protein kinase (MAPK) phosphatase-1-dependent inhibition of p38 MAPK[J]. J Biol Chem 2003; 278: 27811–27819.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Natural Science Foundation of China(No.30100220).
Rights and permissions
About this article
Cite this article
Tong, Xd., Liu, Hx., Zhao, Hr. et al. Cross-talk between Smad4 and P38 proteins in non-small cell lung cancer. Chin. J. Cancer Res. 19, 269–276 (2007). https://doi.org/10.1007/s11670-007-0269-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11670-007-0269-0