Abstract
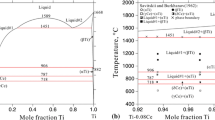
The O-Ti binary system has been assessed to produce Gibbs energy parameters for the condensed phases and were evaluated as representations of thermodynamic models. The liquid phase was described in terms of an association model with one associate, the bcc, A 2; cph, A 3 and fcc, A 1 phases were described as interstitial solid solutions, and the O2Ti, O3Ti5, O3Ti2, and OTi oxides were considered to be stoichiometric compounds. The thermodynamic parameters were optimized taking into account experimental phase diagram and thermodynamic values from the literature. The phase diagram and the thermodynamic properties were calculated and compared with experimental data.
Similar content being viewed by others
Cited References
R.I. Jaffee, H.R. Ogden, and D.J. Maykuth, “Alloys of Titanium with Carbon, Oxygen, Nitrogen,” J. Met., 188(Oct), 1261–1266 (1950).
A.E. Jenkins and H.W. Worner, “The Structure and Some Properties of Titanium-Oxygen Alloys Containing 0-5 at.% Oxygen,” J. Inst. Met., 80, 157–166 (1951–1952).
E.S. Bumps, H.D. Kessler, and M. Hansen, “The Titanium-Oxygen System,” ASM Trans. Q., 45, 1008–1028 (1953).
R.J. Wasilewski and G.L. Kehl, “Diffusion of Nitrogen and Oxygen in Titanium,” J. Inst. Met., 83, 94–104 (1954–1955).
H. Nishimura and H. Kimura, “On the Equilibrium Diagram of Titanium Oxygen-Carbon System, the Titanium-Oxygen System,” J. Jpn. Inst. Met., 20, 524–528 (1956).
T.H. Schofield and A.E. Bacon, “The Constitution of the Titanium-Oxygen Alloys in the Range 0-35 Weight% Oxygen,” J. Inst. Met., 84, 47–53 (1955–1956).
S. Andersson, B. Collen, U. Kuylenstierna, and A. Magneli, “Phase Analysis Studies in the Titanium-Oxygen System,” Acta Chem. Scand., 11(10), 1641–1652 (1957).
A.D. Pearson, “Studies on the Lower Oxides of Titanium,” J. Phys. Chem. Solids, 5(4), 316–327 (1958).
G. Brauer and W. Linke, “Uber den Schmelzpunkt und die thermische dissoziation von titandioxyd,” J. Inorg. Nucl. Chem., 6, 67–76 (1960) in German.
L. Kaufman and E.V. Clougherty, Metallurgy at High Pressures and High Temperatures, AIME Metall. Soc. Conf., Vol. 22, Gordon and Breach Science Publishers, New York, London (1964).
P.G. Wahlbeck and P.W. Gilles, “Reinvestigation of the Phase Diagrams for the System Titanium-Oxygen,” J. Am. Ceram. Soc, 49(4), 180–183 (1966).
M. Hillert and L.I. Staffansson, Acta Chem. Scand., 24, 3618–3626 (1970).
K. Suzuki and K. Sambongi, “High Temperature Thermodynamic Properties in Ti-O System,” Tetsu-to-Hagané, 58, 1579–1593 (1972).
G. Boureau and P. Gerdanian, “Thermodynamic Study of Interstitial Solid Solutions of Oxygen in Titanium at 1050 °C,” Acta Metall., 24, 717–723 (1976).
H.L. Lukas, E.T. Henig, and B. Zimmermann, “Optimization of Phase Diagrams by a Least Squares Method Using Simultaneously Different Types of Data,” Calphad, 1(3), 225–236 (1977).
S.A. Heideman, T.B. Reed, and P.W. Gilles, “The High Temperature Vaporization and Thermodynamics of the Titanium Oxides. XV. Titanium Monoxide Solid Solutions,” High Temp. Sci., 13, 79–90 (1980).
B. Sundman and J. Agren, J. Phys. Chem. Solids, 42, 297–301 (1981).
F. Sommer.Z. Metallkde., 73, 72–86 (1982).
O. Kubaschewski, “Titanium, Physico-Chemical Properties of its Compounds and Alloys,” International Atomic Energy Agency, Vienna (1983).
R. Tetot, C. Picard, and P. Gerdanian, “Determination of Oxygen Partial Free Energy for Non-Stoichiometric TiO by emf Measurements,” J. Phys. Chem. Solids, 44(11), 1059–1068 (1983).
M.W. Chase, Jr., C.A. Davies, J.R. Downey, Jr., D.J. Frurip, R. A. McDonald, and A.N. Syverud, Jr., J. Phys. Chem. Ref. Data, 14, Suppl. No. 1(1985).
J.L. Murray and H.A. Wriedt, “The O-Ti (Oxygen-Titanium) System,” Bull. Alloy Phase Diagrams, 8(2), 148–165 (1987).
A.T. Dinsdale, “SGTE Data for Pure Elements,” Calphad, 15(4), 317–425 (1991).
T.H. Okabe, R.O. Suzuki, T. Oishi, and K. Ono, “Thermodynamic Properties of Dilute Titanium-Oxygen Solid Solution in Beta Phase,” Mater. Trans., JIM, 32(5), 485–488 (1991).
M. Pajunen and J. Kivilahti, “Thermodynamic Analysis of the Ti-OSystem,” Z. Metallkde., 83(1), 17–20 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fischer, E. Thermodynamic calculation of the O-Ti system. JPE 18, 338–343 (1997). https://doi.org/10.1007/s11669-997-0060-4
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11669-997-0060-4