Abstract
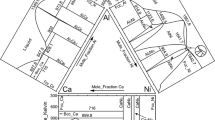
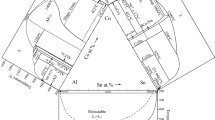
The isothermal sections of the Al-Cr-Mo ternary system at 1200 and 1000 °C were experimentally determined based on microstructure and phase constituents from the equilibrated alloys employing electron probe microanalysis, scanning electron microscopy, and x-ray diffraction. A new ternary compound phase named η with AlTi3-type crystal structure covering a composition range with ~ 75 at.% Al was detected in these two investigated isothermal sections in the present work. Based on the experimental results and the published data of the three binary sub-systems, a thermodynamic description for the Al-Cr-Mo system was carried out using the CALPHAD (CALculation of PHAse Diagrams) method. The newly detected ternary phase η was modeled by a two-sublattice model of (Al)3(Al,Cr,Mo)1. A set of reliable thermodynamic parameters of the Al-Cr-Mo system was obtained, which are in satisfactory agreement with the experimental data. Based on the obtained thermodynamic parameters, much information related to the stable vertical sections, isothermal sections, liquidus projection, and miscibility gap of the bcc phase were predicted in the present work. The present work can provide essential experimental and thermodynamic data for the establishment of the Ni-Al-Cr-Mo based alloy database.












Similar content being viewed by others
References
P. Caron, High γ’ Solvus New Generation Nickel-Based Superalloys for Single Crystal Turbine Blade Applications, Superalloys, 2000, 2000, p 737–746.
H. Fecht and D. Furrer, Processing of Nickel-Base Superalloys for Turbine Engine Disc Applications, Adv. Eng. Mater., 2000, 2(12), p 777–787.
T. Murakumo, T. Kobayashi, Y. Koizumi, and H. Harada, Creep Behaviour of Ni-Base Single-Crystal Superalloys with Various γ′ Volume Fraction, Acta Mater., 2004, 52(12), p 3737–3744.
T.M. Pollock and S. Tin, Nickel-Based Superalloys for Advanced Turbine Engines: Chemistry, Microstructure and Properties, J. Propuls. Power, 2006, 22(2), p 361–374.
R.C. Reed, The Superalloys: Fundamentals and Applications. Cambridge University Press, Cambridge, 2006.
Y.Y. Qiu, Effect of the Al and Mo on the Lattice Mismatch and γ′ Morphology in Ni-Based Superalloys, Scr. Metall. Mater., 1995, 33(12), p 1961–1968.
X. Lu, S. Tian, X. Yu, and C. Wang, Oxidation Behavior of a Single-crystal Ni-base Superalloy in Air at 900 and 1050 °C, Rare Met., 2011, 30(S1), p 439–442.
H. Long, S. Mao, Y. Liu, Z. Zhang, and X. Han, Microstructural and Compositional Design of Ni-Based Single Crystalline Superalloys - A Review, J. Alloys Compd., 2018, 743, p 203–220.
B. Wang, J. Zhang, T. Huang, H. Su, Z. Li, L. Liu, and H. Fu, Influence of W, Re, Cr, and Mo on Microstructural Stability of the Third Generation Ni-Based Single Crystal Superalloys, J. Mater. Res., 2016, 31(21), p 3381–3389.
S.J. Park, S.M. Seo, Y.S. Yoo, H.W. Jeong, and H. Jang, Effects of Cr, W, and Mo on the High Temperature Oxidation of Ni-Based Superalloys, Materials, 2019, 12(18), p 2934.
J.Y. Chen, Q. Feng, L.M. Cao, and Z.Q. Sun, Improvement of Stress-Rupture Property by Cr Addition in Ni-Based Single Crystal Superalloys, Mater. Sci. Eng. A, 2011, 528(10), p 3791–3798.
Q. Shi, J. Huo, Y. Zheng, and Q. Feng, Influence of Mo and Ru Additions on the Creep Behavior of Ni-Based Single Crystal Superalloys at 1100 °C, Mater. Sci. Eng. A, 2018, 725, p 148–159.
B. Seiser, R. Drautz, and D.G. Pettifor, TCP Phase Predictions in Ni-Based Superalloys: Structure Maps Revisited, Acta Mater., 2011, 59(2), p 749–763.
Q. Yu, C. Wang, G. Yang, Y. Ren, N. Liu, Y. Liang, and C. Dong, Influence of Cr/Mo Ratio on Microstructure and Mechanical Properties of the Ni-Based Superalloys Fabricated by Laser Additive Manufacturing, J. Alloys Compd., 2022, 894, p 162484.
B. Grushko, W. Kowalski, D. Pavlyuchkov, B. Przepiórzyński, and M. Surowiec, A Contribution to the Al-Ni-Cr Phase Diagram, J. Alloys Compd., 2008, 460(1), p 299–304.
Y. Wang and G. Cacciamani, Thermodynamic Modeling of the Al-Cr-Ni System over the Entire Composition and Temperature Range, J. Alloys Compd., 2016, 688, p 422–435.
P.E.A. Turchi, L. Kaufman, and Z.K. Liu, Modeling of Ni-Cr-Mo Based Alloys: Part I—Phase Stability, Calphad, 2006, 30(1), p 70–87.
J. Peng. Experimental Investigation and Thermodynamic Modeling of the Al-Cr-Mo-Ni System and Its Sub-Systems, Karlsruher Instituts für Technologie, 2016.
X. Lu, Y. Cui, and Z. Jin, Experimental and Thermodynamic Investigation of the Ni-Al-Mo System, Metall. Mater. Trans. A, 1999, 30(7), p 1785–1795.
S.H. Zhou, Y. Wang, L.Q. Chen, Z.K. Liu, and R.E. Napolitano, Solution-Based Thermodynamic Modeling of the Ni-Al-Mo System Using First-Principles Calculations, Calphad, 2014, 46, p 124–133.
J. Peng, P. Franke, D. Manara, T. Watkins, R.J.M. Konings, and H.J. Seifert, Experimental Investigation and Thermodynamic Re-Assessment of the Al-Mo-Ni System, J. Alloys Compd., 2016, 674, p 305–314.
A. Raman and K. Schubert, Über die Verbreitung des Zr2Cu-Type und Cr2Al-Type, Z. Fuer. Met., 1964, 55(12), p 798–804.
L. Kaufman and H. Nesor, Calculation of Superalloy Phase Diagrams: Part II, Metall. Mater. Trans. B, 1974, 5(7), p 1623–1629.
J. Peng, P. Franke, and H.J. Seifert, Experimental Investigation and CALPHAD Assessment of the Eutectic Trough in the System NiAl-Cr-Mo, J. Phase Equilib. Diffus., 2016, 37(5), p 592–600.
A.T. Dinsdale, SGTE Data for Pure Elements, Calphad, 1991, 15(4), p 317–425.
Y. Liang, C. Guo, C. Li, and Z. Du, Thermodynamic Modeling of the Al-Cr System, J. Alloys Compd., 2008, 460(1), p 314–319.
Z. Du, C. Guo, C. Li, and W. Zhang, Thermodynamic Description of the Al-Mo and Al-Fe-Mo Systems, J. Phase Equilib. Diffus., 2009, 30(5), p 487–501.
K. Frisk and P. Gustafson, An Assessment of the Cr-Mo-W System, Calphad, 1988, 12(3), p 247–254.
O. Redlich and A.T. Kister, Algebraic Representation of Thermodynamic Properties and the Classification of Solutions, Ind. Eng. Chem., 1948, 40(2), p 345–348.
M. Hillert, Empirical Methods of Predicting and Representing Thermodynamic Properties of Ternary Solution Phases, Calphad, 1980, 4(1), p 1–12.
F. Stein, C. He, and I. Wossack, The Liquidus Surface of the Cr-Al-Nb System and Re-Investigation of the Cr-Nb and Al-Cr Phase Diagrams, J. Alloys Compd., 2014, 598, p 253–265.
A. Takeuchi and A. Inoue, Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element, Mater. Trans., 2005, 46(12), p 2817–2829.
O. Kubaschewski and T.G. Chart, Calculation of Metallurgical Equilibrium Diagrams from Thermochemical Data, J. Inst. Met., 1965, 93, p 329–338.
M. Laffitte and O. Kubaschewski, Activities of Chromium in Chromium-Molybdenum Solid Solutions, Trans. Faraday Soc., 1961, 57(6), p 932–934.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant number 51831007), the Shenzhen Science and Technology Program (Grant No. SGDX20210823104002016), and the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2021B1515120071).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Chen, X., Wu, X. et al. Experimental Investigation and Thermodynamic Description of Phase Equilibria in the Al-Cr-Mo Ternary System. J. Phase Equilib. Diffus. 45, 18–35 (2024). https://doi.org/10.1007/s11669-023-01076-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-023-01076-6