Abstract
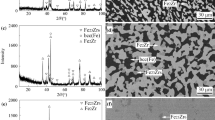
The impact of oxygen impurity on phase relationships in the Zr-Mo-Fe system (Zr > 30 at.%) at 1000 °C was investigated by means of x-ray diffraction, scanning electron microscopy, and energy-dispersive spectroscopy analysis. An oxygen-stabilized ternary compound γ-Zr2(Mo,Fe), derived from Ti2Ni type Zr4Fe2O, with cell parameter of a = 1.2213 nm was observed to be present in the investigated Zr-Mo-Fe alloys. The phase relationships of the Zr-Mo-Fe (O) system at 1000 °C in the Zr-rich corner consist of 3 four-phase regions, i.e., [βZr + λ-Zr(Mo,Fe)2 + γ-Zr2(Mo,Fe) + ZrMo2], [βZr + λ-Zr(Mo,Fe)2 + γ-Zr2(Mo,Fe) + Liquid], and [λ-Zr(Mo,Fe)2 + γ-Zr2(Mo,Fe) + Liquid + ZrFe2]. The previously detected Zr9Mo4Fe compound was not observed in this work.













Similar content being viewed by others
References
A.T. Motta, Waterside Corrosion in Zirconium Alloys, JOM, 2011, 63(8), p 63–67.
J.P. Abriata, J. Garcés, and R. Versaci, The O-Zr (Oxygen-Zirconium) system, Bull. Alloy Phase Diagr., 1986, 1986(7), p 116–124.
M. Billone, Y. Yan, T. Burtseva, R. Daum, Cladding Embrittlement during Postulated Loss-of-Coolant Accidents, NUREG/CR-6967, July 31, 2008; United States. (https://digital.library.unt.edu/ark:/67531/metadc901576/)
G. Hache and H.M. Chung, The History of LOCA Embrittlement Criteria, NUREG/CP-0172, pp. 205-237 (2001)
M. Slobodyan, Arc Welding of Zirconium and Its Alloys: A Review, Prog. Nucl. Energy, 2021, 133, p 103630.
M. Pahutova, K. Kucharova, and J. Cadek, Creep and Creep Fracture in Zr-Sn-Mo and Zr-Sn-Mo-Nb Alloys at Temperatures of 623–823 K—II, Creep Fract. Kovove Mater., 1976, 14(6), p 702–716.
M. Pahutová, J. Čadek, and V. Černý, Creep and Creep Rupture of a Zr-6%Sn-1%Mo Alloy, J. Nucl. Mater., 1977, 68(1), p 111–121.
M. Pahutová, and J. Čadek, Effect of Molybdenum on Some Basic Creep Characteristics of Alpha Zirconium in a Temperature Interval of 350 to 600 °C, Mater. Sci. Eng. C, 1975, 20, p 277–285.
B. Cheadle, R. Holt, V. Fidleris, A. Causey, V. Urbanic, High-strength, creep-resistant excel pressure tubes, zirconium in the nuclear industry. in 4–7 Aug. 1980 (Boston), ASTM Committee B-10 on Reactive and Refractory Metals and Alloys (Sponsor), ed. by D. Franklin, West Conshohocken, PA, ASTM International, pp. 193–207 (1982).
J.H. Lee, and S.K. Hwang, Effect of Mo Addition on the Corrosion Resistance of Zr-Based Alloy in Water Containing LiOH, J. Nucl. Mater., 2003, 321(2), p 238–248.
A.V. Nikulina, V.F. Konkov, M.M. Peregud, and E.E. Vorobev, Effect of Molybdenum on Properties of Zirconium Components of Nuclear Reactor Core, Nucl. Mater. Energy, 2018, 14, p 8–13.
J. Liang, H. Yu, A. Barry, E.C. Corcoran, L. Balogh, and M.R. Daymond, Re-investigation of Phase Transformations in the Zr-Excel Alloy, J. Alloys Compd., 2017, 716, p 7–12.
F. Stein, G. Sauthoff, and M. Palm, Experimental Determination of Intermetallic Phases, Phase Equilibria, and Invariant Reaction Temperatures in the Fe-Zr System, J. Phase Equilib., 2002, 23(6), p 480–494.
R.J. Perez, and B. Sundman, Thermodynamic Assessment of the Mo-Zr Binary Phase Diagram, Calphad, 2003, 27(3), p 253–262.
A.F.A. Guillermet, The Fe-Mo (Iron-Molybdenum) System, Bull. Alloy Phase Diagr., 1982, 3(3), p 359–367.
N.M. Gruzdeva, T.A. Tregubov, ЖEЛEЗO--MOЛИБДEH-ЦИPКOHИЙ (Iron-Molybdenum-Zirconium), Диaгpaммы cocтoяния мeтaлличecкиx cиcтeм (State Diagrams of Metal Systems), Aлиcoвa C.П., Бyдбepг П.Б., Aгeeв H.B. (peд) oпyбликoвaнныe в 1968 гoдy, Bыпycк 14, p 188. (In Russia) Alisova S.P., Budberg P.B., Ageev N.V. (ed), published in 1968, Issue 14, p. 188.
P. Rogl, H. Nowotny, and F. Benesovsky, New K-Borides and Related Phases (Filled up Re3B-Phases), Mon. Chem., 1973, 104(1), p 182–193.
M. Zinkevich, and N. Mattern, Thermodynamic Modeling of the Fe-Mo-Zr System, Acta Mater., 2002, 50(13), p 3373–3383.
Z. Du, L. Zou, C. Guo, X. Ren, and C. Li, Experimental Investigation and Thermodynamic Description of the Fe-Mo-Zr System, Calphad, 2021, 74, p 102314.
A. Boultif, and D.J. Louër, Indexing of Powder Diffraction Patterns for Low-Symmetry Lattices by the Successive Dichotomy Method, J. Appl. Crystallogr., 1991, 24, p 987–993.
A. Boultif, and D. Louër, Powder Pattern Indexing with the Dichotomy Method, J. Appl. Crystallogr., 2004, 37, p 724–731.
D. Louër, and A. Boultif, Indexing with the Successive Dichotomy Method, DICVOL04, Z. Kristallogr. Suppl., 2006, 23, p 225–230.
J. Schindelin, I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair, T. Pietzsch, and A. Cardona, Fiji: An Open-Source Platform for Biological-Image Analysis, Nat. Methods, 2012, 9(7), p 676–682.
International Centre for Diffraction Data, 12 Campus Boulevard, Newtown Square, PA 19073--3273, USA.
A.A. Lavrentyeve, B.V. Gabrelian, P.N. Shkumat, I.Y. Nikiforov, IYu. Zavaliy, and OYu. Khyzhun, Electronic Structure of Zr4Fe2O: Ab Initio APW+LO Calculations and x-ray Spectroscopy Studies, J. Phys. Chem. Solid., 2013, 74, p 590–594.
H. Holleck, and F. Thummler, Ternäre Komplex-Carbide, -Nitride und -Oxide Mit Teilweise Aufgefüllter Ti2Ni-Struktur, Monatsch. Chem., 1967, 98, p 133–134.
R. Mackay, G.J. Miller, and H.F. Franzen, New Oxides of the Filled-Ti2Ni Type Structure, J. Alloys Compd., 1994, 204(1–2), p 109–118.
S. Gupta, D.J. Sordelet, and J.D. Corbett, Structural and Compositional Investigations of Zr4Pt2O: A Filled-Cubic Ti2Ni-Type Phase, J. Solid State Chem., 2009, 182, p 1708–1712.
S. Wang, C. Zhang, C. Lin, Y. Peng, and Y. Du, Measurement of 900 °C Isothermal Section in the Mo-Ni-Zr System, J. Phase Equilib. Diffus., 2016, 37(5), p 672–679.
C. Lin, C. Zhang, S. Wang, P. Zhou, and Y. Du, Phase Equilibria of the Co-Mo-Zr Ternary System at 1000 °C, J. Phase Equilib. Diffus., 2018, 39(5), p 510–518.
J. Sayers, S. Lozano-Perez, and S.R. Ortner, The Progress of SPP Oxidation in Zircaloy-4 and Its Relation to Corrosion and Hydrogen Pickup, Corros. Sci., 2019, 158, p 108972.
C. Proff, S. Abolhassani, and C. Lemaignan, Oxidation Behaviour of Zirconium Alloys and Their Precipitates—A Mechanistic Study, J. Nucl. Mater., 2013, 432(1–3), p 222–238.
Acknowledgment
This project is supported by Guangxi Natural Science Foundation (Grant No. 2018GXNSFAA138043), Open Foundation of Guangxi Key Laboratory of Processing for Non-ferrous Metals and Featured Materials, Guangxi University (Grant No. GXYSOF1803), Guangxi Provincial Science and Technology (Grant No. 2020KY04026, 2022KY0150), Natural Science Foundation of Guangdong Province (2022A1515010919, 2023A1515012089), and the Special Projects of Universities in Guangdong Province in Key Areas (2022ZDZX3014).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, Y., Zhu, J., Liang, J. et al. Phase Relationship in the Zr-Mo-Fe(O) System (Zr > 30 at.%) at 1000 °C. J. Phase Equilib. Diffus. 44, 483–495 (2023). https://doi.org/10.1007/s11669-023-01052-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-023-01052-0