Abstract
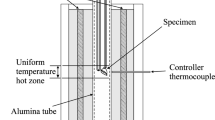
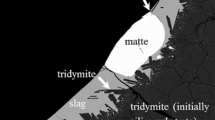
The Cu-Fe-O-S system is the key system for the characterisation of the phase chemistry in high-temperature copper making processes. An experimental study was undertaken to investigate the gas/matte/spinel equilibria in the Cu-Fe-O-S system at 1473 K (1200 °C), P(SO2) = 0.25 atm, and a range of oxygen partial pressures. The experimental methodology involved high temperature equilibration using a primary phase substrate technique in controlled gas atmospheres (CO/CO2/SO2/Ar), rapid quenching of the equilibrated phases, followed by direct measurement of phase compositions using electron probe x-ray microanalysis. Particular attention was given to the analysis of reactions during equilibration and confirmation of the achievement of equilibrium in the present study. The new data provide important information for understanding of the gas/matte/spinel interactions at high temperature and provide an essential foundation for the development of the multicomponent thermodynamic database for copper-containing systems.











Similar content being viewed by others
References
F.Y. Bor and P. Tarassoff, Can. Metall. Q., 1971, 10, p 267-271
A. Geveci and T. Rosenqvist, Trans. Inst. Min. Metall., 1973, 82, p C193-C201
M. Nagamori, Metall. Trans. B, 1974, 5B, p 531-538
U. Kuxmann and H. Bussman, Erzmetall, 1974, 27, p 353-365
G.H. Kaiura, K. Watanabe, and A. Yazawa, Can. Metall. Q., 1980, 19, p 191-200
H. Jalkanen, Scand. J. Metall., 1981, 10, p 177-184
A. Yazawa, S. Nakazawa, and Y. Takeda, Distribution Behavior of Various Elements in Copper Smelting Systems. in Proceedings of International Sulphide Smelting Symposium, Metall. Soc. AIME, San Francisco, USA, 1983, p 99–117
H.K. Jalkanen, L.E.K. Holappa, and J.K. Makinen, Some Novel Aspects of Matte-Slag Equilibria. in Proceedings of International Sulphide Smelting Symposium, Metall. Soc. AIME, San Francisco, USA, 1983, p 277–292
R. Shimpo, S. Goto, O. Ogawa, and I. Asakura, Can. Metall. Q., 1986, 25, p 113-121
F.J. Tavera and E. Bedolla, Int. J. Miner. Process., 1990, 29, p 289-309
A. Yazawa and M. Kameda, Technol. Rep. Tohoku Univ., 1955, 19, p 251-261
N. Korakas, Etude Thermodynamic de L’équilibre Entre Escories Ferro-Siliceuses et Mattes de Cuivre. Application aux Problèmes Posés par la Formation de Magnetite lors du Traitement des Minerais Sulfurés de Cuivre, Vaillant-Carmanne, Águas de Lindóia, 1964
U. Kuxmann and F.Y. Bor, Erzmetall, 1965, 18, p 441-450
F.J. Tavera and W.G. Davenport, Metall. Trans. B, 1979, 10B, p 237-241
H. Li and W.J. Rankin, Metall. Trans. B, 1994, 25B, p 79-89
Y. Takeda, Oxygen Potential Measurement of Iron Silicate Slag-Copper-Matte System. in Proceedings of the 5th International Conference on Molten Slags, Fluxes and Salts, Iron and Steel Society, Sydney, Australia, 1997, p 735–743
G. Roghani, M. Hino, and K. Itagaki, Mater. Trans., JIM, 1997, 38, p 707-713
J.M. Font, G. Roghani, M. Hino, and K. Itagaki, Metall. Rev. MMIJ, 1998, 15, p 75-86
G. Roghani, Y. Takeda, and K. Itagaki, Metall. Mater. Trans. B, 2000, 31B, p 705-712
H.M. Henao, L.A. Ushkov, and E. Jak, Thermodynamic Predictions and Experimental Investigation of Slag Liquidus and Minor Element Partitioning Between Slag and Matte in Support of the Copper Isasmelt Smelting Process Commissioning and Optimisation at Kazzinc. in Proceedings of the 9th International Conference on Molten Slags, Fluxes and Salts (Molten12), The Chinese Society for Metals, 2012, Paper No. W078
Z. Sun, T. Hidayat, P. Hayes, and E. Jak, Liquidus Temperatures, Major and Minor Elements Equilibrium Partitioning in Copper Smelting Slag/Matte/Gas Systems. in Proceedings of the 8th International Copper Conference, Chilean Institute of Mining Engineers (IIMCh), Santiago, Chile, 2013, p 33–44
H.N. Lander, Thesis, Massachusetts Institute of Technology, USA, 1954 cited in Ref. [26]
T. Rosenqvist and T. Hartvig, Norges Tek.-Naturvitenskapelige Forskiningsrud Met. Kom. Meddel, 1958, 24, p 21-52
F. Johannsen and H. Knahl, Z. Erzbergbau Metallhuettenwes, 1963, 16, p 611-621
M. Kameda and A. Yazawa, Stud. Met. 1969, p 159-166.
A. Luraschi and J.F. Elliott, Trans. Inst. Min. Metall., 1980, 89, p C14-C25
D.L. Kaiser and J.F. Elliott, Metall. Trans. B, 1988, 19, p 935-941
E. Jak, S.A. Decterov, P.C. Hayes, and A.D. Pelton, Thermodynamic Modelling of the System PbO-ZnO-FeO-Fe2O3-CaO-SiO2 for Zinc/Lead Smelting. in Proceedings of the 5th International Conference on Molten Slags, Fluxes and Salts, Iron and Steel Society, Sydney, Australia, 1997, p 621–628
S.A. Degterov and A.D. Pelton, Metall. Mater. Trans. B, 1999, 30B, p 661-669
E. Jak, S.A. Decterov, B. Zhao, A.D. Pelton, and P.C. Hayes, Metall. Mater. Trans. B, 2000, 31B, p 621-630
S.A. Decterov, I.-H. Jung, E. Jak, Y.-B. Kang, P.C. Hayes, and A.D. Pelton, Thermodynamic Modeling of the Al2O3-CaO-CoO-CrO-Cr2O3-FeO-Fe2O3-MgO-MnO-NiO-SiO2-S System and Applications in Ferrous Process Metallurgy. in The 7th International Conference on Molten Slags, Fluxes and Salts, The South African Institute of Mining and Metallurgy, Cape Town, Republic of South Africa, 2004, p 839–850
FactSage 7.0, FToxid and FTmisc Databases, 2015. http://www.factsage.com/
E. Jak, P.C. Hayes, and H.-G. Lee, Met. Mater. (Seoul), 1995, 1, p 1-8
E. Jak, Integrated Experimental and Thermodynamic Modelling Research Methodology for Metallurgical Slags with Examples in the Copper Production Field. in The 9th International Conference on Molten Slags, Fluxes and Salts (Molten12), The Chinese Society for Metals, Beijing, China, 2012, Paper No. W077
A. Fallah-Mehrjardi, T. Hidayat, P.C. Hayes, and E. Jak, Metall. Mater. Trans. B, 2017, 48, p 3002-3016
T. Hidayat, D. Shishin, E. Jak, and S. Decterov, Calphad, 2015, 48, p 131-144
A. Fallah-Mehrjardi, T. Hidayat, P.C. Hayes, and E. Jak, Metall. Mater. Trans. B, 2017, 48, p 3017-3026
Acknowledgments
The authors would like to thank the Australian Research Council Linkage program LP140100480, Altonorte Glencore, Atlantic Copper, Aurubis, BHP Billiton Olympic Dam Operation, Kazzinc Glencore, PASAR Glencore, Outotec Oy (Espoo), Anglo American Platinum, Umicore, and Rio Tinto Kennecott for the financial and technical support for this research. The authors acknowledge the support of the AMMRF at the Centre for Microscopy and Microanalysis at the University of Queensland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hidayat, T., Hayes, P.C. & Jak, E. Experimental Investigation of Gas/Matte/Spinel Equilibria in the Cu-Fe-O-S System at 1473 K (1200 °C) and P(SO2) = 0.25 atm. J. Phase Equilib. Diffus. 39, 138–151 (2018). https://doi.org/10.1007/s11669-018-0616-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-018-0616-5