Abstract
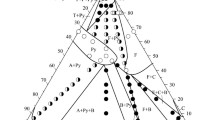
Phase equilibria in the ZrO2-Nd2O3-Y2O3 system at 1523-1873 K have been investigated by x-ray diffraction (XRD) and scanning electron microscopy combined with energy dispersive x-ray spectroscopy (SEM/EDX). Temperatures of phase transformations were determined by differential thermal analysis. Temperatures of invariant reactions in the ZrO2-Nd2O3 system F = A + Pyr and H = F + A were determined as 1763 and 2118 K respectively and thermodynamic parameters of phases were re-assessed. Phase transformations in ternary systems were determined at 1732 K for composition ZrO2-48.46Nd2O3-5.38Y2O3 (mol%) and at 1744 and 1881 K for composition ZrO2-79.09Nd2O3-2.75Y2O3 (mol%). They were interpreted using XRD investigation before and after DTA as Pyr + B → F, Pyr → F and A → B, respectively. The solubility of the Y2O3 in pyrochlore phase was found to exceed 10 mol%. The thermodynamic parameters of the ZrO2-Nd2O3-Y2O3 system were reassessed taking into account solubility of Y2O3 in the Nd2Zr2O7 pyrochlore phase (Pyr). It is assumed that Y3+ substitutes Nd3+ and Zr4+ in their preferentially occupied sublattices. Ternary parameter was introduced into fluorite phase (F) for better reproducing of phase equilibria. Mixing parameters were reassessed for phase A (Nd2O3 based solution), monoclinic phase B and cubic phase C (Y2O3 based solution). The isothermal sections calculated for the ZrO2-Nd2O3-Y2O3 system are in the reasonable agreement with experimental results.








Similar content being viewed by others
References
C.G. Levi, Emerging Materials and Processes for Thermal Barrier System, Curr. Opin. Solid State Mater. Sci., 2004, 8, p 77-91
N.R. Rebollo, A.S. Gandhi, and C.G. Levi, Phase Stability Issues in Emerging TBC Systems, High Temperature Corrosion and Materials Chemistry IV, Electrochemical Society Proceedings, Vol. PV-2003-16, E.J. Opila, P. Hou, T. Maruyama, B. Pieraggi, M. McNallan, D. Shifler, and E. Wuchina, Ed., 2003, p 431-442
K.A. Khor and J. Yang, Rapidly Solidified Neodymia-Stablised Zirconia Coatings Prepared by DC Plasma Spaying, Surf. Coat. Technol., 1997, 96, p 313-322
T. Xu, J. Vleugels, O. Van der Biest, Y. Kann, and P. Wang, Phase Stability and Mechanical Properties of TZP with a Low Mixed Nd2O3/Y2O3 Stabiliser Content, J. Eur. Ceram. Soc., 2006, 26, p 1205-1211
Q. Xu, W. Pan, J. Wang, Ch. Wan, L. Qi, and H. Mia, Rare Earth Zirconate Ceramics with Fluorite Structure for Thermal Barrier Coatings, J. Am. Ceram. Soc., 2006, 89, p 340-342
T. Xu, J. Vleugels, O. Van der Biest, and P. Wang, Mechanical Properties of Nd2O3/Y2O3-Coated Zirconia Ceramics, Mater. Sci. Eng. A, 2004, 374, p 239-243
Y. Hinatsu and T. Muromura, Phase Relations in the Systems ZrO2-Y2O3-Nd2O3 and ZrO2-Y2O3-CeO2, Mater. Res. Bull., 1986, 21, p 1343-1349
S. Lutique, R.J.M. Konings, V.V. Rondinella, J. Somers, and T. Wiss, The Thermal Conductivity of Nd2Zr2O7 Pyrochlore and the Thermal Behaviour of Pyrochlore-Based Inert Matrix Fuel, J. Alloys Compd., 2003, 352, p 1-5
O. Fabrichnaya, G. Savinykh, G. Schreiber, and H.J. Seifert, Phase Relations in the ZrO2-Nd2O3-Y2O3 System: Experimental Study and CALPHAD Assessment, Int. J. Mater. Res., 2010, 101, p 1354-1360
B.P. Mandal, P.S.R. Krishna, and A.K. Tyagi, Order-Disorder Transition in the Nd2-yYyZr2O7 System: Probed by X-ray Diffraction and Raman Spectroscopy, J. Solid State Chem., 2010, 183, p 41-45
M. Hillert, Compound Energy Formalism, J. Alloys Compd., 2001, 320, p 161-176
O. Fabrichnaya and H.J. Seifert, Assessment of Thermodynamic Functions in the ZrO2-Nd2O3-Al2O3 System, CALPHAD, 2008, 3, p 142-151
O. Fabrichnaya, G. Savinykh, G. Schreiber, M. Dopita, and H.J. Seifert, Experimental Investigation and Thermodynamic Modelling in the ZrO2-La2O3-Y2O3 System, J. Alloys Compd., 2010, 493, p 263-271
O. Fabrichnaya, Ch. Wang, M. Zinkevich, C.G. Levi, and F. Aldinger, Phase Equilibria and Thermodynamic Properties of the ZrO2-GdO1.5-YO1.5 System, J. Phase Equilibria Diffus., 2005, 26, p 591-604
O. Fabrichnaya, M. Zinkevich, and F. Aldinger, Thermodynamic Modelling in the ZrO2-La2O3-Y2O3-Al2O3 System, Int. J. Mater. Res., 2007, 98, p 838-846
A. Navrotsky, Thermodynamic Insights into Refractory Ceramic Materials Based on Oxides with Large Tetravalent Cations, J. Mater. Chem., 2005, 15, p 1883-1890
A. Rouanet, High Temperature Properties of Zirconia-Neodimium Sesquioxide Oxide Systems, C. R. Acad. Sci. Paris, Ser. C, 1970, t.270(9), p 802-805
Ch. Wang, “Experimental and Computational Phase Studies of the ZrO2-Based Systems for Thermal Barrier Coatings”, Ph.D. Thesis, Stuttgart University, Germany, 2006
I.A. Davtyan, V.B. Glushkova, and E.K. Keler, A Study of the Nd2O3-ZrO2 System. Investigation of the Regions Rich in Zirconium Dioxide, Inorg. Mater., 1965, 1(5), p 679-685
V.B. Glushkova, I.A. Davtyan, and E.K. Keler, Study of the System Nd2O3-ZrO2. Investigation of Regions Rich in Neodymium Oxide, Inorg. Mater., 1965, 1(11), p 1766-1774
A. Rouanet, Contribution to Study of Zirconium Oxides of Lanthanides Close to Melting Point, Rev. Int. Hautes Temp. Refract., 1971, 8, p 161-180
A.M. Gavrish, N.V. Gul’ko, and L.A. Tarasova, X-ray Diffraction and Crystal-Optical Investigation of Phase Relations in the Nd2O3-ZrO2 System, Russ. J. Inorg. Chem., 1981, 26, p 1785-1788
A.M. Gavrish, L.S. Alekseenko, L.A. Tarasova, and G.P. Orekhova, Structure and Electrical Resistance of Solid Solutions in the ZrO2-Nd2O3 System, Inorg. Mater., 1982, 18(2), p 214-216
E.I. Zoz, E.N. Fomichev, A.A. Kalashnik, and G.G. Eliseeva, The Structure and Properties of Lanthanide Zirconates and Hafnates, Russ. J. Inorg. Chem., 1982, 27, p 54-56
E.R. Andrievskaya and L.M. Lopato, Influence of Composition on the TM Transformation in the System ZrO2-Ln2O3 (Ln = La, Nd, Sm, Eu), J. Mater. Sci., 1995, 30, p 2591-2596
J. Katamura, T. Seri, and T. Sakuma, The Cubic-Tetragonal Phase Equilibria in the System ZrO2-R2O3 (R = Y, Gd, Sm, Nd) System, J. Phase Equilibria, 1995, 16, p 315-319
R.M. Leckie, S. Kraemer, M. Ruhle, and C.G. Levi, Thermochemical Compatibility Between Alumina and ZrO2-GdO3/2 Thermal Barrier Coatings, Acta Mater., 2005, 53, p 3281-3292
M. Zinkevich, Thermodynamics of Rare Earth Sesquioxides, Prog. Mater. Sci., 2007, 52, p 597-647
A. Dinsdale, SGTE Data for Pure Elements, CALPHAD, 2001, 15, p 317-425
Acknowledgment
This study was financially supported by DFG project SE 647/9-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fabrichnaya, O., Savinykh, G., Schreiber, G. et al. Phase Relations in the ZrO2-Nd2O3-Y2O3 System: Experimental Study and Advanced Thermodynamic Modeling. J. Phase Equilib. Diffus. 32, 284–297 (2011). https://doi.org/10.1007/s11669-011-9903-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-011-9903-0