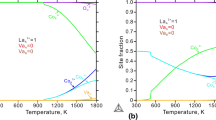
The La-Fe and the La-Fe-O systems are assessed using the Calphad approach, and the Gibbs energy functions of ternary oxides are presented. Oxygen and mutual La and Fe solubilities in body-centered cubic (bcc) and face-centered cubic (fcc) structured metallic phases are considered in the modeling. Oxygen nonstoichiometry of perovskite-structured La1±x Fe1±y O3−δ is modeled using the compound energy formalism (CEF), and the model is submitted to a defect chemistry analysis. The contribution to the Gibbs energy of LaFeO3 due to a magnetic order-disorder transition is included in the model description. Lanthanum-doped hexaferrite, LaFe12O19, is modeled as a stoichiometric phase. Δf,elements°H 298 K (LaFe12O19) = −5745 kJ/mol, °S 298 K (LaFe12O19) = 683 J/mol · K, and Δf,oxides°G (LaFe12O19) = 4634 − 37.071T (J/mol) from 1073 to 1723 K are calculated. The liquid phase is modeled using the two-sublattice model for ionic liquids. The calculated La-Fe phase diagram, LaO1.5-FeO x phase diagrams at different oxygen partial pressures, and phase equilibria of the La-Fe-O system at 873, 1073, and 1273 K as a function of oxygen partial pressures are presented.












Similar content being viewed by others
References
L.V. Goncharuk, V.R. Sidorko, Thermodynamics of Interaction of Rare-Earth Metals with d-Metals. The Scandium—Iron System, Powder Metall. Met. Ceram., 2001, 40(7-8), p 354-361.
E.M. Savitskii, Investigation of the Physico-Chemical Interactions of Rare-Earth Metals with Iron and Steel, Proc. Conf. Rare Earth Elements for Steel and Alloys, 1959, p 31-49.
J. Linden, “Austenitic Stainless Steel with High Oxidation Resistance,” Patent DE69813156T2 06.11.2003, EP-number: 0921206. www.patent-de.com/20031106/DE69813156T2.html.
M. Küpferling, V.C. Flores, R. Grössinger, and M. Aquino, Preparation and Characterization of LaFe12O19 Hexaferrite, J. Magn. Magn. Mater., 2005, 290-291, p 1255-1258.
A.M. Van Diepen, F.K. Lotgering, Mössbauer Effect in LaFe12O19, J. Phys. Chem. Solids, 1974, 35, p 1641-1643.
E. Pollert, Crystal Chemistry of Magnetic Oxides Part 2: Hexagonal Ferrites, Prog. Cryst. Growth Charact., 1985, 11, p 155-205.
K.A. Gschneidner Jr., Rare Earth Alloys, A Critical Review of the Alloy Systems of the Rare Earth, Scandium and Yttrium Metals, D. Van Nostrand Company, Princeton, NJ, 1961, p 187-188.
F.H. Spedding and A.H. Daane, Ed., The Rare Earths, John Wiley & Sons, New York, 1961, p 280, 415-416
J. Richerd, Lanthanum and Cerium in Pure Iron, Mem. Sci. Rev. Metall., 1962, 59(7-8), p 539-548 (in French).
M. Kepka, J. Skala, Effect of Rare-Earth Elements on Properties of Steels, Hutník, 1972, 22(1), p 12-17 (in Czech).
K. Nassau, L.V. Cherry, W.E. Wallace, Intermetallic Compounds between Lanthanons and Transition Metals of the First Long Period. I-Preparation, Existence and Structural Studies, J. Phys. Chem. Solids, 1960, 16, p 123-130.
J.F. Cannon, D.L. Robertson, H.T. Hall, Synthesis of Lanthanide-Iron Laves Phases at High Pressures and Temperatures, Mater. Res. Bull., 1972, 7, p 5-12.
E.M. Savitskii, Rare Metals and Alloys, Dom Tekhniki, Moscow, 1959, in Russian. Cited by Ref 11
E.M. Savitskii, Rare-Earth Metals, Metalloved. Term. Obrab. Met., 1961, 9, p 28 (in Russian).
W. Zhang, C. Li, The Fe-La (Iron-Lanthanum) System, J. Phase Equilib., 1997, 18(3), p 301-304.
V.V. Berezutskii, N.I. Usenko, and M.I. Ivanov (2006) Thermochemistry of Binary Alloys of Lanthanum with 3d-Transition Metals, Powder Metall Met Ceram., 45(5-6): 266-271.
Y.O. Esin, A.F. Ermakov, M.G. Valishev, G.M. Ryss, P.V. Geld, and E.S. Levin, Enthalpy of Formation of Liquid Binary Alloys of Iron with Lanthanum and Cerium, Zh. Fiz. Khim., 1981, 55(7), p 1665-1669, in Russian.
H. Okamoto, Thermodynamically Improbable Phase Diagrams, J. Phase Equilib., 1991, 12(2), p 148-168.
H. Okamoto, Phase Diagrams of Binary Iron Alloys, ASM International, Materials Park, OH, 1993, p 341-349.
V.L. Moruzzi and M.W. Shafer, Phase Equilibria in the System La2O3-Iron Oxide in Air, J. Am. Ceram. Soc., 1960, 43(7), p 367-372.
J. Cassedanne, H. Forestier (1960) Investigation of the Systems Fe2O3-La2O3 and Fe2O3-Sc2O3, C.R. Acad. Sci. (Paris), 250: 2898-2900 (in French).
M. Kowalski, P.J. Spencer, Thermodynamic Reevaluation of the Cr-O, Fe-O and Ni-O Systems: Remodelling of the Liquid, BCC and FCC Phases, Calphad, 1995, 19(3), p 229-243.
A.N. Grundy, B. Hallstedt, L.J. Gauckler, Thermodynamic Assessment of the Lanthanum-Oxygen System, J. Phase Equilib., 2001, 22(2), p 105-113.
T. Nakamura, G. Petzow, L.J. Gauckler, Stability of the Perovskite Phase LaBO3 (B = V, Cr, Mn, Fe, Co, Ni) in Reducing Atmosphere I. Experimental Results, Mater. Res. Bull., 1979, 14, p 649-659.
S. Stølen, F. Grønvold, H. Brinks, T. Atake, and H. Mori, Heat Capacity and Thermodynamic Properties of LaFeO3 and LaCoO3 from T = 13 K to T = 1000 K, J. Chem. Thermodyn., 1998, 30, p 365-377.
J. Cheng and A. Navrotsky, Enthalpies of Formation of LaMO3 Perovskites (M = Cr, Fe, Co, and Ni), J. Mater. Res., 2005, 20(1), p 191-200.
S. Tanasescu, N.D. Totir, D.I. Marchidan, Thermodynamic Properties of LaFeO3 Studied by Means of Galvanic Cells with Solid Oxide Electrolyte, Mater. Res. Bull., 1997, 32(7), p 925-931.
O.M. Sreedharan, M.S. Chandrasekharaiah, Standard Gibbs’ Energy of Formation of LaFeO3 and Comparison of Stability of LaMO3 (M = Mn, Fe, Co or Ni) Compounds, J. Mater. Sci., 1986, 21, p 2581-2584.
Y.D. Tretyakov, A.R. Kaul, V.K. Portnoy, Formation of Rare Earth and Yttrium Orthoferrites: A Thermodynamic Study, High Temp. Sci., 1977, 9, p 61-70.
S.C. Parida, Z. Singh, S. Dash, R. Prasad, V. Venugopal, Thermodynamic Studies on LaFeO3(s), J. Alloys Compd., 1998, 280, p 94-98.
T. Katsura, T. Sekine, K. Kitayama, T. Sugihara, Thermodynamic Properties of Fe-Lanthanoid-O Compounds at High Temperatures, J. Solid State Chem., 1978, 23, p 43-57.
T. Sugihara, Thesis of D.Sc., Department of Chemistry, Faculty of Science, Tokyo Institute of Technology, Meguro-ku, Tokyo, Japan, 1979, p 152, in Japanese
N. Kimizuka, T. Katsura, The Standard Free Energy of the Formation of LaFeO3 at 1204°C, Bull. Chem. Soc. Jpn., 1974, 47(7), p 1801-1802.
S.A. Leontev, Y.P. Vorobev, A.M. Balbechor, A.N. Men, A.Y. Cherronenkis, G.I. Chufaov, Thermodynamic Properties of Orthoferrites of Rare-Earth Elements and Yttrium, Dokl. Akad. Nauk USSR, 1973, 209, p 618-620 (in Russian).
J. Mizusaki, T. Sasamoto, W.R. Cannon, H.K. Bowen, Electronic Conductivity, Seebeck Coefficient, and Defect Structure of LaFeO3, J. Am. Ceram. Soc., 1982, 65(8), p 363-368.
A. Fossdal, M. Menon, I. Waernhus, K. Wiik, M.-A. Einarsrud, and T. Grande, Crystal Structure and Thermal Expansion of La1−x Sr x FeO3−δ Materials, J. Am. Ceram. Soc., 2004, 87(10), p 1952-1958.
S. Geller, P.M. Raccah, Phase Transitions in Perovskitelike Compounds of the Rare Earths, Phys. Rev. B, 1970, 2(4), p 1167-1172.
J. Mizusaki, M. Yoshihiro, S. Yamauchi, K. Fueki, Nonstoichiometry and Defect Structure of the Perovskite-Type Oxides La1−x Sr x FeO3−δ, J. Solid State Chem., 1985, 58, p 257-266.
I. Waernhus, P.E. Vullum, R. Holmestad, T. Grande, K. Wiik, Electronic Properties of Polycrystalline LaFeO3. Part I: Experimental Results and the Qualitative Role of Schottky Defects, Solid State Ionics, 2005, 176, p 2783-2790.
I. Waernhus, T. Grande, K. Wiik, Electronic Properties of Polycrystalline LaFeO3. Part II: Defect Modelling Including Schottky Defects, Solid State Ionics, 2005, 176(35-36), p 2609-2616.
M. Zinkevich, S. Geupel, F. Aldinger, A. Durygin, S.K. Saxena, M. Yang, Z.-K. Liu, Phase Diagram and Thermodynamics of the La2O3-Ga2O3 System Revisited, J. Phys. Chem. Solids, 2006, 67, p 1901-1907.
M. Selleby, B. Sundman, A Reassessment of the Ca-Fe-O System, Calphad, 1996, 20(3), p 381-392.
J.R. Taylor, A.T. Dinsdale, A Thermodynamic Assessment of the Cr-Fe-O System, Z. Metallkd., 1993, 84, p 335-345.
A.T. Dinsdale, SGTE Data for Pure Elements, Calphad, 1991, 15(4), p 317-425.
B. Hallstedt, N. Dupin, M. Hillert, L. Höglund, H.L. Lukas, J.C. Schuster, N. Solak, Thermodynamic Models for Crystalline Phases. Composition Dependent Models for Volume, Bulk Modulus and Thermal Expansion, Calphad, 2007, 31(1), p 28-37.
B. Sundman, B. Jansson, J.-O. Andersson, The Thermo-Calc Databank System, Calphad, 1985, 9(2), p 153-190.
M.T. Hepworth, R.P. Smith, E.T. Turkdogan, Permeability Solubility and Diffusivity of Oxygen in Bcc Iron, Trans. AIME, 1966, 236, p 1278.
J.H. Swisher, E.T. Turkdogan, Solubility Permeability and Diffusivity of Oxygen in Solid Iron, Trans. AIME, 1967, 239, p 426-431.
M. Chen, B. Hallstedt, L.J. Gauckler, Thermodynamic Assessment of the Co-O System, J. Phase Equilib., 2003, 24(3), p 212-227.
N. Saunders and A.P. Miodownik, Calphad Calculation of Phase Diagrams, Pergamon Materials Series, Vol 1. Elsevier Science Ltd., Oxford, UK, 1998, p 94-96
B.C. Tofield, W.R. Scott, Oxidative Nonstoichiometry in Perovskite, an Experimental Survey; the Defect Structure of an Oxidized Lanthanum Manganite by Powder Neutron Diffraction, J. Solid State Chem., 1974, 10, p 183-194.
P. Porta, S. Cimino, S. De Rossi, M. Faticanti, G. Minelli, I. Pettiti, AFeO3 (A = La, Nd, Sm) and LaFe1−x Mg x O3 Perovskites: Structural and Redox Properties, Mater. Chem. Phys., 2001, 71, p 165-173.
P. Ciambelli, S. Cimino, S. De Rossi, L. Lisi, G. Minelli, P. Porta, and G. Russo, AFeO3 (A = La, Nd, Sm) and LaFe1−x Mg x O3 Perovskites as Methane Combustion and CO Oxidation Catalysts: Structural, Redox and Catalytic Properties, Appl. Catal. B-Environ., 2001, 29, p 239-250.
J.-O. Andersson, A.F. Guillermet, M. Hillert, B. Jansson, and B. Sundman, A Compound-Energy Model of Ordering in a Phase with Sites of Different Coordination Numbers, Acta Metall., 1986, 34, p 437-445.
M. Hillert, B. Jansson, B. Sundman, Application of the Compound-Energy Model to Oxide Systems, Z. Metallkd., 1988, 79(2), p 81-87.
M. Hillert, The Compound Energy Formalism, J. Alloys Compd., 2001, 320, p 161-176.
A.N. Grundy, M. Chen, B. Hallstedt, L.J. Gauckler, Assessment of the La-Mn-O System, J. Phase Equilib. Diff., 2005, 26(2), p 131-151.
G. Inden, Determination of Chemical and Magnetic Interchange Energies in BCC Alloys. I. General Treatment, Z. Metallkd., 1975, 66(10), p 577-582.
M. Hillert, M. Jarl, A Model of Alloying Effects in Ferromagnetic Metals, Calphad, 1978, 2(3), p 227-238.
A.N. Grundy, E. Povoden, T. Ivas, L.J. Gauckler, Calculation of Defect Chemistry Using the CALPHAD Approach, Calphad, 2005, 30, p 33-41.
A. Deschamps, F. Bertaut, On the Substitution of Al, Ga, and Cr with Fe in Baryum Hexaferrite BaO.6Fe2O3, C.R. Acad. Sci. (Paris), 1957, 244, p 3069-3072 (in French).
M. Hillert, B. Jansson, B. Sundman, J. Ågren, A Two-Sublattice Model of Molten Solutions with Different Tendency of Ionization, Metall. Trans. A, 1985, 16A, p 261-266.
B. Sundman, Modification of the Two-Sublattice Model for Liquids, Calphad, 1991, 15, p 109-119.
R. Ferro, G. Borzone, G. Cacciamani, and N. Parodi, Thermodynamics of Rare Earth Alloys: Systematics and Experimental, Themochim. Acta, 1998, 314(1-2), p 183-204.
E. Povoden, A.N. Grundy, M. Chen, T. Ivas and L.J. Gauckler, Thermodynamic Assessment of the La-Sr-Fe-O System, in preparation.
Acknowledgment
This work was financially supported by the Federal Agency for Education and Science, Sixth Framework Program for Research and Technical Development of the European Union.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Povoden-Karadeniz, E., Grundy, A.N., Chen, M. et al. Thermodynamic Assessment of the La-Fe-O System. J. Phase Equilib. Diffus. 30, 351–366 (2009). https://doi.org/10.1007/s11669-009-9501-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-009-9501-6