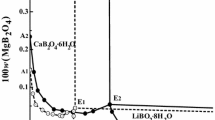
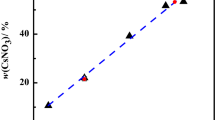
The solubility data of the calcium acetate-magnesium acetate-water system at 298, 313, and 323 K were measured using the Schreinemaker’s wet residue method, the corresponding phase diagram for the system were constructed. The solid phase in the system at different temperatures was confirmed by the Schreinemaker’s wet residue method, which correspond to Mg(CH3COO)2·4H2O and Ca(CH3COO)2·2H2O. At the studied temperature, no double salt was formed. The crystalline region of Ca(CH3COO)2·2H2O is larger than that of Mg(CH3COO)2·4H2O. The solubility of Mg(CH3COO)2 increases with the increasing of the temperature, while the solubility of Ca(CH3COO)2 decreases with the increasing of the temperature.



Similar content being viewed by others
References
Y.A. Levendis, W. Zhu, D.L. Wise, G.A. Simons, Effectiveness of Calcium Magnesium Acetate as an SOx Sorbent in Coal Combustion, AIChE J., 1993, 39(3), 761-773, in English
J.I. Shuckerow, J.A. Steciak, D.L. Wise, Y.A. Levendis, G.A. Simons, J.D. Gresser, E.B. Gutoff, C.D. Livengood, Control of Air Toxin Particulate and Vapor Emissions after Coal Combustion Utilizing Calcium Magnesium Acetate, Resour. Conserv. Recy., 1996, 16(1), 15-69, in English
J. Steciak, Y.A. Levendis, D.L. Wise, Effectiveness of Calcium Magnesium Acetate as Dual SO2–NOx Emission Control Agent, AIChE J., 1995, 41(3), 712-722, in English
A. Ergut, Y.A. Levendis, G.A. Simons, High Temperature Injection of Sorbent-Coal Blends Upstream of a Ceramic Filter for SO2, NOx and Particulate Pollutant Reductions, Combust Sci. Technol., 2003, 175(3), 579-617, in English
W. Nimmo, J. Agnew, E. Hampartsoumian, J.M. Jones, H2S Removal Using Spray-Pyrolysed Calcium Acetate, Ind. Eng. Chem. Res., 1999, 38(8), 2954-2962, in English
F. Garcia-Labiano, L.F. de Diego, V. Fierro, Utilization of Calcium Acetate and Calcium Magnesium Acetate for H2S Removal in Coal Gas Cleaning at High Temperatures, Energ. Fuel, 1999, 13(2), p 440–448, in English
A. Ohtsuka, K. Asami, In-bed Sulfur Removal during the Fluidised Bed Combustion of Coal Impregnated with Calcium Magnesium Acetate, Resour. Conserv. Recycl., 1992, 7(1), p 69-82, in English
B.A. Gancy, Process of Making Calcium Acetate Deicing Agents, U.S. patent 4,426,308, January 17, 1984, in English
B.A. Gancy, Process of Making Calcium Acetate Deicing Agents and Product, U.S. patent 4,444,672, April 24, 1984, in English
B.A. Gancy, Nonpolluting Salts and Method of Making Same, U.S. patent 4,511,485, April 16, 1985, in English
B.A. Gancy, Continuous Process for the Manufacture of Calcium Magnesium Acetate Deicer, U.S. patent 4,606,836, April 19, 1986, in English
S. Fu, C. Hercules, Process for Manufacturing Crystalline Calcium Magnesium Acetate, U.S. patent 5,430,185, July 4, 1995, in English
D.D. Dionysiou, M. Tsianou, G.D. Botsaris, Investigation of the Conditions for the Production of Calcium Magnesium Acetate (CMA) Road Deicer in an Extractive Crystallization Process, Cryst. Res. Technol., 2000, 35(9), 1035-1049, in English
E.R. Krasnicki, Löslichkeitsbestimmung der Kalk- und Barytsalze, der Ameisensäure, Essigsäure und Propionsäure, Monatsh. Chem., 1887, 8(1), p 595-606, in German
J.S. Lumsden, Solubilities of the Calcium Salts of the Acids of the Acetic Series, J. Chem. Soc., 1902, 81(2), 355-362, in English
L.J. Dunn, J.C. Philip, Acid Salts in Systems of the Type Monobasic Acid–Alkaline-Earth Salt–Water, J. Chem. Soc., 1934, 113(3), p 658-666, in English
C. Saury, R. Boistelle, F. Dalemat, J. Bruggeman, Solubilities of Calcium Acetates in the Temperature Range 0–100 Degree.C, J. Chem. Eng. Data., 1993, 38(1), p56-59, in English
A. Apelblat, E. Manzurola, Solubilities of Magnesium, Calcium, Barium, Cobalt, Nickel, Copper, and Zinc Acetates in Water from T = (278.15 to 348.15) K, J. Chem. Thermodyn., 1999, 31(10), 1347-1357, in English
A.C.D. Rivett, The Constitution of Magnesium Acetate Solutions, J. Chem. Soc., 1926, 105(5), 1063-1070, in English
A. Apelblat Solubilities of Organic Salts of Magnesium, Calcium, and Iron in Water, J. Chem. Thermodyn., 1993, 25(12), p 1443-1445, in English
Acknowledgments
We thank the High Education Natural Science Foundation of Jiangsu Province (Grant No. HK051087) and the Open Project Program of the Key Laboratory of Physical Chemistry, Yangzhou University, China for their support. We are also grateful to the editors and the anonymous referee for their valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, HK., Zhang, DS., Tang, C. et al. Phase Diagram of Ternary Calcium Acetate—Magnesium Acetate—Water System at 298 K, 313 K and 323 K. J Phs Eqil and Diff 28, 167–171 (2007). https://doi.org/10.1007/s11669-007-9020-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-007-9020-2