Abstract
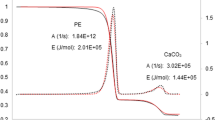
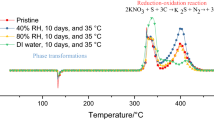
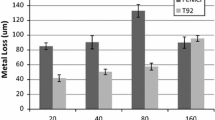
A recent article (Casey et al. in J Failure Anal Prev 22:1252–1259, 2022) finds a thermodynamic explanation for the catastrophic effect of water as an extinguisher in aluminum-clad tower block fires (Entropy 22: 14, 2020) to be “unsubstantiated hypotheses” and “suppositions unsupported by data”. The article by Casey et al., however, is misleading because it is based upon a false premise that it is the hydrolysis of solid aluminum panels that produce hydrogen (H2), which was not detected in their experiments. The combustion of aluminum (Al) to alumina (Al2O3) reaction is highly exothermic to the extent that it can be explosive, but the reaction is inhibited, for all temperatures of solid and liquid Al, below the melting point of alumina (2250 °C), by the formation of a thin nanometre skin of alumina that prevents combustion. In tower block infernos of Al-plastic cladding materials, there cannot be production of detectable hydrogen gas, as wrongly assumed by Casey et al., who only investigate the laboratory hydrolysis reaction of solid aluminum in cladding samples, and not the combustion conditions at temperatures exceeding 1500 °C. Here we show that H2 is an intermediate in the Al-combustion mechanism of cladding fires, and that water (H2O) is the catalyst. This is one of two possible reaction mechanisms that enable combustion by circumventing direct oxidation of Al. For cladding with adjacent plastic insulation material, water provides an alternative mechanism via methane and the carbide Al4C3 as intermediates, also with H2O as the catalyst.


Similar content being viewed by others
References
L. Casey, S. Simandjuntak, J. Zekonyte, J.M. Buick, A. Saifullah, Investigating the effects of H2O interaction with rainscreen facade ACMs during fire exposure. J. Fail. Anal. Preven. 22, 1252–1259 (2022). https://doi.org/10.1007/s11668-022-01417-6
J.F. Maguire, L.V. Woodcock, Thermodynamics of tower block infernos: effects of water on aluminium fires entropy 22, 1–14 (2019) https://www.mdpi.com/1099-4300/22/1/14/pdf
J. Petrovic, G. Thomas. Reaction of aluminum with water to produce hydrogen US Department of Energy White Paper (2008) version 1.0 1–26
I.E. Smith, Hydrogen generation by means of the aluminum/water reaction. J. Hydronaut. 6(2), 106–109 (1972)
1 H.J. Richter, K. F. Knoche. Chemical Combustion Looping Efficiency and Costing, American Chemical Society, ch. 3, 235, 71–85 (1983)
X. Zhu, Q. Imtiaz, Chemical looping beyond combustion—a perspective. Energy Environ. Sci. 13(3), 155 (2020). https://doi.org/10.1039/C9EE03793D
Wikipedia: “catalysis” https://en.wikipedia.org/wiki/Catalysis
S.I. Sandler, L.V. Woodcock, Historical observations of the laws of thermodynamics. J. Chem. Eng. Data. 55, 4485–4490 (2010)
Y. Lu. X. Wang, Y. Zhang, J. Wang, M.J. Kim. Aluminium Carbide hydrolysis induced degradation of thermal conductivity and tensile strength in diamond/aluminum composite Journal of Composite Materials 52, 20 (2018) https://www.researchgate.net/publication/323001846/
B. Novak, K. Tschöpe, A.P. Ratvik, T. Grande Fundamentals of Aluminum Carbide Formation Light Metals 2012 Chapter 23.2 Editor(s): Carlos E. Suarez (Wiley: March 2012) https://doi.org/10.1002/9781118359259.ch232
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maguire, J.F., Woodcock, L.V. On the Thermodynamics of Aluminum Cladding Oxidation: Water as the Catalyst for Spontaneous Combustion. J Fail. Anal. and Preven. 22, 1771–1775 (2022). https://doi.org/10.1007/s11668-022-01471-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-022-01471-0