Abstract
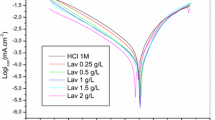
The essential oil obtained from the aerial parts of Aaronsohnia pubescens plant (AP oil) was extracted by hydrodistillation, and then, its composition was analyzed by gas chromatography (GC) and GC-mass spectrometry (GC/MS). The corrosion inhibition and adsorption performance of the AP oil on the corrosion mild steel in 1 M hydrochloric acid was evaluated by weight loss analysis, electrochemical methods both stationary [Potentiodynamic polarization (PDP)] and transient [Electrochemical impedance spectroscopy (EIS)]. The weight loss outcomes indicate that AP oil shows a worthy inhibitory efficiency of 83.22% reached at 1.5 g L−1 and 343 K. The charge transfer process mainly controls the results of EIS measurements; PDP measurements showed that the studied AP oil performs as a mixed-type inhibitor. Furthermore, the adsorption on the steel is made according to the Langmuir model. The computational chemistry calculations by density functional theory, the electrostatic potential surface and Metropolis Monte Carlo methods were performed to acquire a greater understanding of adsorption mechanism for each compound among the third major compounds of AP oil on the Fe (110) surface. The finding results exhibited that the E-anethole will be considered as the best inhibitor against mild steel corrosion.












Similar content being viewed by others
References
A. Sedik, D. Lerari, A. Salci, S. Athmani, K. Bachari, İ.H. Gecibesler, R. Solmaz, Dardagan Fruit extract as eco-friendly corrosion inhibitor for mild steel in 1 M HCl: electrochemical and surface morphological studies. J. Taiwan Inst. Chem. Eng. 107, 189–200 (2020)
C.A. Loto, R.T. Loto, Influence of Lavandula latifolia and Ricinus communis oils on the corrosion control of mild steel in HCl solution. J. Fail. Anal. Prev. 19, 1853–1859 (2019)
S. Lahrour, A. Benmoussat, B. Bouras, A. Mansri, L. Tannouga, S. Marzorati, Glycerin-grafted starch as corrosion inhibitor of C-Mn steel in 1 M HCl solution. Appl. Sci. 9(21), 4684 (2019)
D.K. Lavanya, F.V. Priya, D.P. Vijaya, Green approach to corrosion inhibition of mild steel in hydrochloric acid by 1-[Morpholin-4-yl (thiophen-2-yl) methyl] thiourea. J. Fail. Anal. Prev. 20, 494–502 (2020)
M.Y. El Sayed, A.M. Abdel-Gaber, H.T. Rahal, Safranin—a potential corrosion inhibitor for mild steel in acidic media: a combined experimental and theoretical approach. J. Fail. Anal. Prev. 19, 1174–1180 (2019)
G.A. Swetha, H.P. Sachin, A.M. Guruprasad, B.M. Prasanna, Rizatriptan Benzoate as corrosion inhibitor for mild steel in acidic corrosive medium: experimental and theoretical analysis. J. Fail. Anal. Prev. 19, 1113–1126 (2019)
S. Rajendraprasad, S. Ali, B.M. Prasanna, Electrochemical behavior of N1-(3 methylphenyl)piperidine-1,4-dicarboxamide as a corrosion inhibitor for soft-cast steel carbon steel in 1 M HCl. J. Fail. Anal. Prev. 20, 235–241 (2020)
I. Merimi, Y. Ouadi, R. Benkaddour, H. Lgaz, M. Messali, F. Jeffali, B. Hammouti, Improving corrosion inhibition potentials using two triazole derivatives for mild steel in acidic medium: experimental and theoretical studies. Mater. Today Proc. 13, 920–930 (2019)
Y.E. Louadi, F. Abrigach, A. Bouyanzer, R. Touzani, A. ElAssyry, Theoretical and experimental studies on the corrosion inhibition potentials of two Tetrakis pyrazole derivatives for mild steel in 1.0 M HCl. Port. Electrochim. Acta. 35(3), 159–178 (2017). https://doi.org/10.4152/pea.201703159
A.S. Fouda, A.H. El-Azaly, R.S. Awad, A.M. Ahmed, New benzonitrile azo dyes as corrosion inhibitors for carbon steel in hydrochloric acid solutions. Int. J. Electrochem. Sci. 9, 1117–1131 (2014)
J.H. Al-Fahemi, M. Abdallah, E.A.M. Gad, B. Jahdaly, Experimental and theoretical approach studies for melatonin drug as safely corrosion inhibitors for carbon steel using DFT. J. Mol. Liq. 222, 1157–1163 (2016)
M. Manssouri, Y. El Ouadi, M. Znini, J. Costa, A. Bouyanzer, J.M. Desjobert, L. Majidi, Adsorption proprieties and inhibition of mild steel corrosion in HCl solution by the essential oil from fruit of Moroccan Ammodaucus leucotrichus. J. Mater. Environ. Sci. 6(3), 631–646 (2015)
M. Manssouri, M. Znini, A. Ansari, A. Bouyanzer, Z. Faska, L. Majidi, Odorized and deodorized aqueous extracts of Ammodaucus leucotrichus fruits as green inhibitor for C38 steel in hydrochloric acid solution. Der Pharma. Chem. 6(6), 331–345 (2014)
M. Manssouri, M. Znini, Z. Lakbaibi, Y. ElOuadi, L. Majidi, Aqueous extracts of Santolina pectinata lag., aerial parts as green corrosion inhibitor for mild steel in 1.0 M HCl. Anal. Bioanal. Electrochem. 12(5), 607–624 (2020)
M. Manssouri, A. Laghchimi, A. Ansari, M. Znini, Z. Lakbaibi, Y. ElOuadi, L. Majidi, Effect of Santolina pectinata (Lag.) essential oil to protect against the corrosion of mild steel in 1.0 M HCl: experimental and quantum chemical studies. Mediterr. J. Chem. 10(3), 253–268 (2020). https://doi.org/10.13171/mjc02003171332mm
S. Benhouhou, Aaronsohnia pubescens (Dasf.) K. Bremer & Humphries. A Guide to Medicinal Plants in North Africa, Malaga (Spain), IUCN Centre for Mediterranean Cooperation (2005)
J.A. Reyes-Betancort, S. Scholz, M.C.L. Arencibia, About the presence of the Moroccan endemic Aaronsohnia pubescens subsp. maroccana in the Canary Islands (Anthemidae, Asteraceae). Vieraea, p 233–236 (2003)
M. Abouri, A. El Mousadik, F. Msanda, H. Boubaker, B. Saadi, K. Cherifi, An ethnobotanical survey of medicinal plants used in the Tata Province, Morocco. Int. J. Med. Plants Res. 1(7), 99–123 (2012)
M. Djellouli, A. Moussaoui, H. Benmehdi, L. Ziane, A. Belabbes, M. Badraoui, N. Slimani, N. Hamidi, Ethnopharmacological study and phytochemical screening of three plants (Asteraceae family) from the region of South West Algeria. Asian J. Nat. Appl. Sci. 2, 59–65 (2013)
C. Verma, L.O. Olasunkanmi, I.B. Obot, E.E. Ebenso, M.A. Quraishi, 2, 4-Diamino-5-(phenylthio)-5 H-chromeno [2, 3-b] pyridine-3-carbonitriles as green and effective corrosion inhibitors: gravimetric, electrochemical, surface morphology and theoretical studies. RSC Adv. 6(59), 53933–53948 (2016)
C. Verma, H. Lgaz, D.K. Verma, E.E. Ebenso, I. Bahadur, M.A. Quraishi, Molecular dynamics and Monte Carlo simulations as powerful tools for study of interfacial adsorption behavior of corrosion inhibitors in aqueous phase: a review. J. Mol. Liq. 260, 99–120 (2018)
Council of Europe, E. Pharmacopoeia, European Pharmacopoeia: Supplement. Council of Europe (1998)
Standard, A.S.T.M. G1-03. Standard practice for preparing, cleaning, and evaluating corrosion test specimens. Annual Book of ASTM Standards, p 17–25 (2003)
R. Nabah, F. Benhiba, Y. Ramli, M. Ouakki, M. Cherkaoui, H. Oudda, I. Warad, A. Zarrouk, Corrosion inhibition study of 5, 5-diphenylimidazolidine-2, 4-dione for mild steel corrosion in 1 M HCl solution: experimental, theoretical computational and Monte Carlo simulations studies. Anal. Bioanal. Electrochem. 10(10), 1375–1398 (2018)
A. Boumezzourh, M. Ouknin, E. Chibane, J. Costa, A. Bouyanzer, B. Hammouti, L. Majidi, Inhibition of tinplate corrosion in 0.5 M H2C2O4 medium by Mentha pulegium essential oil. Int. J. Corros. Scale Inhib. 9(1), 152–170 (2020)
A. Zarrouk, H. Zarrok, Y. Ramli, M. Bouachrine, B. Hammouti, A. Sahibed-Dine, F. Bentiss, Inhibitive properties, adsorption and theoretical study of 3, 7-dimethyl-1-(prop-2-yn-1-yl) quinoxalin-2 (1H)-one as efficient corrosion inhibitor for carbon steel in hydrochloric acid solution. J. Mol. Liq. 222, 239–252 (2016)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, Gaussian 09 revision D. 01, 2009, Gaussian Inc, Wallingford, CT, p 93 (2009)
P.C. Hariharan, J.A. Pople, The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 28(3), 213–222 (1973)
W.J. Hehre, R. Ditchfield, J.A. Pople, Self-consistent molecular orbital methods. XII. Further extensions of Gaussian—type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 56(5), 2257–2261 (1972)
R. Hssissou, B. Benzidia, N. Hajjaji, A. Elharfi, Elaboration, electrochemical investigation and morphological study of the coating behavior of a new polymeric polyepoxide architecture: crosslinked and hybrid decaglycidyl of phosphorus penta methylene dianiline on E24 carbon steel in 3.5% NaCl. Port. Electrochim. Acta 37(3), 179–191 (2019)
P. Geerlings, F. De Proft, W. Langenaeker, Conceptual density functional theory. Chem. Rev. 103(5), 1793–1874 (2003)
K. Vanasundari, V. Balachandran, M. Kavimani, B. Narayana, Spectroscopic investigation, vibrational assignments, Fukui functions, HOMO-LUMO, MEP and molecular docking evaluation of 4–[(3, 4–dichlorophenyl) amino] 2–methylidene 4–oxo butanoic acid by DFT method. J. Mol. Struct. 1147, 136–147 (2017)
A. Benallou, Z. Lakbaibi, H. Garmes, H.E.A. El Abdallaoui, The role of the polarity on the mechanism and selectivity in the [3 + 2] cycloaddition reaction between CF3-ynone ylide and azide group: a quantum chemical investigation. J. Fluor. Chem. 219, 79–91 (2019)
S. Martinez, Inhibitory mechanism of mimosa tannin using molecular modeling and substitutional adsorption isotherms. Mater. Chem. Phys. 77(1), 97–102 (2003)
R.G. Parr, R.G. Pearson, Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 105(26), 7512–7516 (1983)
Y. Tang, F. Zhang, S. Hu, Z. Cao, Z. Wu, W. Jing, Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part I: gravimetric, electrochemical, SEM and XPS studies. Corros. Sci. 74, 271–282 (2013)
A. Kokalj, On the HSAB based estimate of charge transfer between adsorbates and metal surfaces. Chem. Phys. 393(1), 1–12 (2012)
L.O. Olasunkanmi, M.M. Kabanda, E.E. Ebenso, Quinoxaline derivatives as corrosion inhibitors for mild steel in hydrochloric acid medium: electrochemical and quantum chemical studies. Phys. E Low Dimens. Syst. Nanostruct. 76, 109–126 (2016)
C.M. Goulart, A. Esteves-Souza, C.A. Martinez-Huitle, C.J.F. Rodrigues, M.A.M. Maciel, A. Echevarria, Experimental and theoretical evaluation of semicarbazones and thiosemicarbazones as organic corrosion inhibitors. Corros. Sci. 67, 281–291 (2013)
H. Sun, COMPASS: an ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. J. Phys. Chem. B 102, 7338–7364 (1998)
Accelrys Software Inc., Materials Studio Revision 8.0. San Diego San Diego, CA, USA (2006)
M. Shahraki, M. Dehdab, S. Elmi, Theoretical studies on the corrosion inhibition performance of three amine derivatives on carbon steel: molecular dynamics simulation and density functional theory approaches. J. Taiwan Inst. Chem. Eng. 62, 313–321 (2016)
L. Messaadia, O. IdElmouden, A. Anejjar, M. Messali, R. Salghi, O. Benali, O. Cherkaoui, A. Lallam, Adsorption and corrosion inhibition of new synthesized pyridazinium-based ionic liquid on carbon steel in 0.5 M H2SO4. J. Mater. Environ. Sci. 6(2), 598–606 (2015)
S. Martinez, I. Stern, Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in the low carbon steel/mimosa tannin/sulfuric acid system. Appl. Surf. Sci. 199(1–4), 83–89 (2002)
A.M. Badiea, K.N. Mohana, Effect of temperature and fluid velocity on corrosion mechanism of low carbon steel in presence of 2-hydrazino-4, 7-dimethylbenzothiazole in industrial water medium. Corros. Sci. 51(9), 2231–2241 (2009)
S. Kharchouf, L. Majidi, M. Znini, J. Costa, B. Hammouti, J. Paolini, Stereoselective synthesis and corrosion inhibition behaviour of Allyldihydrocarveols on steel in molar hydrochloric acid. Int. J. Electrochem. Sci. 7, 10325–10337 (2012)
I. El Ouali, B. Hammouti, A. Aouniti, Y. Ramli, M. Azougagh, E.M. Essassi, M. Bouachrine, Thermodynamic characterisation of steel corrosion in HCl in the presence of 2-phenylthieno (3, 2-b) quinoxaline. J. Mater. Environ. Sci. 1(1), 1–8 (2010)
Y. El Ouadi, A. Bouyanzer, L. Majidi, J. Paolini, J.-M. Desjobert, J. Costa, A. Chetouani, B. Hammouti, S. Jodeh, I. Warad, Evaluation of Pelargonium extract and oil as eco-friendly corrosion inhibitor for steel in acidic chloride solutions and pharmacological properties. Res. Chem. Intermed. 41(10), 7125–7149 (2015)
Y. Kharbach, F.Z. Qachchachi, A. Haoudi, M. Tourabi, A. Zarrouk, C. Jama, L.O. Olasunkanmi, E.E. Ebenso, F. Bentiss, Anticorrosion performance of three newly synthesized is a tin derivatives on carbon steel in hydrochloric acid pickling environment: electrochemical, surface and theoretical studies. J. Mol. Liq. 246, 302–316 (2017)
A.R. SathiyaPriya, V.S. Muralidharan, A. Subramania, Development of novel acidizing inhibitors for carbon steel corrosion in 15% boiling hydrochloric acid. Corrosion 64(8), 541–552 (2008)
K. Jüttner, Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochim. Acta 35(10), 1501–1508 (1990)
D.K. Yadav, M.A. Quraishi, B. Maiti, Inhibition effect of some benzylidenes on mild steel in 1 M HCl: an experimental and theoretical correlation. Corros. Sci. 55, 254–266 (2012)
A.K. Satapathy, G. Gunasekaran, S.C. Sahoo, K. Amit, P.V. Rodrigues, Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros. Sci. 51(12), 2848–2856 (2009)
F. El-Hajjaji, M. Messali, A. Aljuhani, M.R. Aouad, B. Hammouti, M.E. Belghiti, D.S. Chauhan, M.A. Quraishi, Pyridazinium-based ionic liquids as novel and green corrosion inhibitors of carbon steel in acid medium: electrochemical and molecular dynamics simulation studies. J. Mol. Liq. 249, 997–1008 (2018)
Acknowledgements
The authors extend their appreciation to the Moroccan Association of theoretical chemists (AMCT) for access to the computational facility.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manssouri, M., Lakbaibi, Z., Znini, M. et al. Impact of Aaronsohnia pubescens Essential Oil to Prevent Against the Corrosion of Mild Steel in 1.0 M HCl: Experimental and Computational Modeling Studies. J Fail. Anal. and Preven. 20, 1939–1953 (2020). https://doi.org/10.1007/s11668-020-01003-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-020-01003-8