Abstract
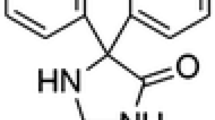
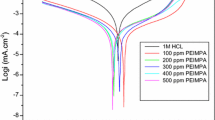
The importance of the use of various drugs as corrosion inhibitors has developed. In this context, the corrosion inhibition of C38 steel in 1.0 M H3PO4 medium by omeprazole has been evaluated by weight loss tests, potentiodynamic polarization and electrochemical impedance spectroscopy measurements. The surface morphology has been characterized by scanning electron microscopy coupled with energy-dispersive x-ray spectroscopy. The Tafel polarization analysis shows that omeprazole is a mixed-type inhibitor. The results obtained by electrochemical impedance spectroscopy indicate that the inhibition efficacy increases with inhibitor concentration. The results obtained from the standard free energy of adsorption of \((\Delta {G}_{\text{ads}}^{0}=- 37.86\) kJ mol−1) are consistent with a physisorption and chemisorption process. The scanning electron microscopy allowed the visualization of a protective layer on the C38 steel surface after the addition of the inhibitor. The results obtained from different tested techniques showed excellent inhibition efficiency up to 99% using omeprazole for C38 steel in a phosphoric acid medium.







Similar content being viewed by others
References
C. Verma, E.E. Ebenso, M.A. Quraishi, Ionic liquids as green and sustainable corrosion inhibitors for metals and alloys: an overview. J. Mol. Liq. 233, 403–414 (2017). https://doi.org/10.1016/j.molliq.2017.02.111
T.Q. Ansari, J. Luo, S. Shi, Modeling the effect of insoluble corrosion products on pitting corrosion kinetics of metals. NPJ Mater. Degrad. (2019). https://doi.org/10.1038/s41529-019-0090-5
P.B. Raja, M.G. Sethuraman, Natural products as corrosion inhibitor for metals in corrosive media—a review. Mater. Lett. 62, 113–116 (2007). https://doi.org/10.1016/j.matlet.2007.04.079
T. Lipinski, Corrosion effect of 20% NaCl solution on basic carbon structural S235JR steel, in 16th International Scientific Conference: Engineering for Rural Development, vol. 2 (2017), pp. 1069–1074. https://doi.org/10.22616/ERDev2017.16.N225
N.G. Thompson, D. Ph, M. Yunovich, Cost of corrosion and corrosion maintenance strategies. Corros. Rev. 25, 247–262 (2007)
S.A. Umoren, M.M. Solomon, Effect of halide ions on the corrosion inhibition efficiency of different organic species—a review. J. Ind. Eng. Chem. 21, 81–100 (2015). https://doi.org/10.1016/j.jiec.2014.09.033
C. Verma, J. Haque, M.A. Quraishi, E.E. Ebenso, Aqueous phase environmental friendly organic corrosion inhibitors derived from one step multicomponent reactions: a review. J. Mol. Liq. (2018). https://doi.org/10.1016/j.molliq.2018.11.040
M. Tariq, M. Saleem, S. Usmani, I. Ahmed, F.A. Al-shammari, K. Mairaj, Inhibition of mild steel in 1 M HCl by sweet melon peel extract. J. King Saud. Univ. Sci. (2019). https://doi.org/10.1016/j.jksus.2019.01.013
P.B. Raja, M. Ismail, S. Ghoreishiamiri, Reviews on corrosion inhibitors—a short view. Chem. Eng. Commun. (2016). https://doi.org/10.1080/00986445.2016.1172485
G. Karthik, M. Sundaravadivelu, Investigations of the inhibition of copper corrosion in nitric acid solutions by Levetiracetam drug. Egypt. J. Pet. (2015). https://doi.org/10.1016/j.ejpe.2015.10.009
L.T. Popoola, Progress on pharmaceutical drugs, plant extracts and ionic liquids as corrosion inhibitors. Heliyon (2019). https://doi.org/10.1016/j.heliyon.2019.e01143
G. Gece, Drugs: a review of promising novel corrosion inhibitors. Corros. Sci. 53, 3873–3898 (2011). https://doi.org/10.1016/j.corsci.2011.08.006
A. Kokalj, Is the analysis of molecular electronic structure of corrosion inhibitors sufficient to predict the trend of their inhibition performance. Electrochim. Acta. 56, 745–755 (2010). https://doi.org/10.1016/j.electacta.2010.09.065
X. Luo, S. Zhang, L. Guo, Investigation of a pharmaceutically active compound omeprazole as inhibitor for corrosion of mild steel in H2SO4 solution. Int. J. Electrochem. Sci. 9, 7309–7324 (2014)
A.A. Fadhil, A.A. Khadom, H. Liu, C. Fu, J. Wang, N.A. Fadhil, H.B. Mahood, Corrosion inhibitor of steel in acidic solution: gravimetrical, electrochemical, surface morphology and theoretical simulation. J. Mol. Liq. 276, 503–518 (2019). https://doi.org/10.1016/j.molliq.2018.12.015
H. So, A. Saxena, D. Prasad, R. Haldhar, Á.S.E.M.Á. Afm, Investigation of corrosion inhibition effect and adsorption activities of Achyranthes aspera extract for mild steel in 0.5 M. J. Fail. Anal. Prev. (2018). https://doi.org/10.1007/s11668-018-0491-8
A. Khadraoui, A. Khelifa, M. Hadjmeliani, R. Mehdaoui, K. Hachama, A. Tidu, Z. Azari, Extraction, characterization and anti-corrosion activity of Mentha pulegium oil: weight loss, electrochemical, thermodynamic and surface studies. J. Mol. Liq. 216, 724–731 (2016). https://doi.org/10.1016/j.molliq.2016.02.005
Q. Hu, Y. Qiu, G. Zhang, X. Guo, Capsella bursa-pastoris extract as an eco-friendly inhibitor on the corrosion of Q235 carbon steels in 1 mol L−1 hydrochloric acid. Chin. J. Chem. Eng. (2015). https://doi.org/10.1016/j.cjche.2015.05.002
F.Z. Bouanis, F. Bentiss, M. Traisnel, C. Jama, Enhanced corrosion resistance properties of radiofrequency cold plasma nitrided carbon steel: gravimetric and electrochemical results. Electrochim. Acta. 54, 2371–2378 (2009). https://doi.org/10.1016/j.electacta.2008.10.068
M. Elfaydy, H. Lgaz, R. Salghi, M. Larouj, S. Jodeh, Investigation of corrosion inhibition mechanism of quinoline derivative on mild steel in 1.0 M HCl solution: experimental, theoretical and Monte Carlo simulation. J. Mater. Environ. Sci. 7, 3193–3210 (2016)
C. Machado, S.F. Fagundes, N. Escarpini, T. Shewry, D.M. Rocha, R. Garrett, R. Moreira, G. Muricy, A. Leda, E. Ariel, Ircinia strobilina crude extract as corrosion inhibitor for mild steel in acid medium. Electrochim. Acta. 312, 137–148 (2019). https://doi.org/10.1016/j.electacta.2019.04.148
A. Maxime, C. Florent, R. Nadine, M. Traisnel, C. Roos, M. Lebrini, Enhanced corrosion resistance of mild steel in 1 M hydrochloric acid solution by alkaloids extract from Aniba rosaeodora plant: electrochemical, phytochemical and XPS studies. Electrochim. Acta. (2013). https://doi.org/10.1016/j.electacta.2013.12.023
M. Messali, H. Lgaz, R. Dassanayake, R. Salghi, S. Jodeh, N. Abidi, O. Hamed, Guar gum as efficient non-toxic inhibitor of carbon steel corrosion in phosphoric acid medium: electrochemical, surface, DFT and MD simulations studies. J. Mol. Struct. (2017). https://doi.org/10.1016/j.molstruc.2017.05.081
N. Soltani, N. Tavakkoli, M. Khayatkashani, M. Reza, Green approach to corrosion inhibition of 304 stainless steel in hydrochloric acid solution by the extract of Salvia officinalis leaves. Corros. Sci. 62, 122–135 (2012). https://doi.org/10.1016/j.corsci.2012.05.003
S. Cao, D. Liu, H. Ding, J. Wang, H. Lu, J. Gui, Task-specific ionic liquids as corrosion inhibitors on carbon steel in 0.5 M HCl solution: an experimental and theoretical study. Corros. Sci. 153, 301–313 (2019). https://doi.org/10.1016/j.corsci.2019.03.035
Q.H. Zhang, B.S. Hou, Y.Y. Li, G.Y. Zhu, H.F. Liu, G.A. Zhang, Two novel chitosan derivatives as high efficient eco-friendly inhibitors for the corrosion of mild steel in acidic solution. Case Stud. Fire Saf. (2019). https://doi.org/10.1016/j.corsci.2019.108346
I. Nadi, Z. Belattmania, B. Sabour, A. Reani, A. Sahibed-dine, C. Jama, F. Bentiss, Sargassum muticum extract based on alginate biopolymer as a new efficient biological corrosion inhibitor for carbon steel in hydrochloric acid pickling environment: gravimetric, electrochemical and surface studies. Int. J. Biol. Macromol. 141, 1–13 (2019). https://doi.org/10.1016/j.ijbiomac.2019.08.253
I.B. Onyeachu, I. Bassey, A.A. Sorour, M.I. Abdul-rashid, Green corrosion inhibitor for oil field application I: electrochemical assessment of 2-(2-pyridyl)benzimidazole for API X60 steel under sweet environment in NACE brine ID196. Corros. Sci. 150, 183–193 (2019). https://doi.org/10.1016/j.corsci.2019.02.010
R. Sadeghi, M. Amirnasr, S. Meghdadi, M. Talebian, Carboxamide derivatives as new corrosion inhibitors for mild steel protection in hydrochloric acid solution. Corros. Sci. 151, 190–197 (2019). https://doi.org/10.1016/j.corsci.2019.02.019
Y. Ye, D. Yang, H. Chen, S. Guo, Q. Yang, L. Chen, H. Zhao, L. Wang, A high-efficiency corrosion inhibitor of N-doped citric acid-based carbon dots for mild steel in hydrochloric acid environment. J. Hazard. Mater. (2020). https://doi.org/10.1016/j.jhazmat.2019.121019
F.Z. Bouanis, C. Jama, M. Traisnel, F. Bentiss, Study of corrosion resistance properties of nitrided carbon steel using radiofrequency N2/H2 cold plasma process. Corros. Sci. 52, 3180–3190 (2010). https://doi.org/10.1016/j.corsci.2010.05.021
W. Xu, E. Han, Z. Wang, Effect of tannic acid on corrosion behavior of carbon steel in NaCl solution. J. Mater. Sci. Technol. 35, 64–75 (2019). https://doi.org/10.1016/j.jmst.2018.09.001
A.H. Alamri, I.B. Obot, Highly efficient corrosion inhibitor for C1020 carbon steel during acid cleaning in multistage flash (MSF) desalination plant. Desalination (2019). https://doi.org/10.1016/j.desal.2019.114100
J. Prakash, R. Shrivastava, R. Kumar, Corrosion inhibition effect of Clerodendron colebrookianum Walp leaves (Phuinam) extract on the acid corrosion of mild steel. Prot. Metals Phys. Chem. Surf. (2018). https://doi.org/10.1134/S2070205118010264
I.R. Saad, A.M. Abdel-Gaber, G.O. Younes, B. Nsouli, Thiourea and N-methylthiourea as corrosion inhibitors for steel in phosphoric acid. J. Fail. Anal. Prevent. (2018). https://doi.org/10.1007/s11668-018-0522-5
K.R.R.S. Bhat, Inhibition effects of ethyl-2-amino-4-methyl-1,3-thiazole-5-carboxylate on the corrosion of AA6061 alloy in hydrochloric acid media. J. Fail. Anal. Prev. (2019). https://doi.org/10.1007/s11668-019-00744-5
M. El, B. Lakhrissi, C. Jama, A. Zarrouk, Electrochemical, surface and computational studies on the inhibition performance of some newly synthesized 8-hydroxyquinoline derivatives containing benzimidazole moiety against the corrosion of carbon steel in phosphoric acid. J. Mater. Res. Technol. (2019). https://doi.org/10.1016/j.jmrt.2019.11.014
F. Bentiss, M. Lebrini, H. Vezin, F. Chai, M. Traisnel, M. Lagrené, Enhanced corrosion resistance of carbon steel in normal sulfuric acid medium by some macrocyclic polyether compounds containing a 1,3,4-thiadiazole moiety : AC impedance and computational studies. Corros. Sci. 51, 2165–2173 (2009). https://doi.org/10.1016/j.corsci.2009.05.049
F. Suedile, F. Robert, C. Roos, M. Lebrini, Corrosion inhibition of zinc by Mansoa alliacea plant extract in sodium chloride media: extraction, characterization and electrochemical studies. Electrochim. Acta. (2013). https://doi.org/10.1016/j.electacta.2013.12.070
M. Larouj, H. Lgaz, H. Serrar, H. Zarrok, H. Bourazmi, A. Zarrouk, A. Elmidaoui, A. Guenbour, S. Boukhris, H. Oudda, Adsorption properties and inhibition of carbon steel corrosion in hydrochloric acid solution by ethyl 3-hydroxy-8-methyl-4-oxo-6-phenyl-2- (p-toly)-4,6-dihydropyrimido [2,1-b][1,3] thiazine-7-carboxylate. J. Mater. Environ. Sci. 6, 3251–3267 (2015)
M. El Azzouzi, A. Aouniti, S. Tighadouin, H. Elmsellem, S. Radi, B. Hammouti, A. El Assyry, F. Bentiss, A. Zarrouk, Some hydrazine derivatives as corrosion inhibitors for mild steel in 1.0 M HCl weight loss, electrochemichal, SEM and theoretical studies. J. Mol. Liq. (2016). https://doi.org/10.1016/j.molliq.2016.06.007
R.G. Sundaram, M.T.G. Vengatesh, Surface protection and morphological study of copper by green corrosion inhibitor: 8QSC in HNO3 medium. J. Fail. Anal. Prev. (2018). https://doi.org/10.1007/s11668-018-0506-5
K.K.J. Ravichandran, A study on the inhibition of copper corrosion in sulphuric acid by aqueous extract of leaves of Morinda tinctoria. J. Fail. Anal. Prev. 15, 711–721 (2015). https://doi.org/10.1007/s11668-015-0002-0
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Hamdouni, Y., Bouhlal, F., Kouri, H. et al. Use of Omeprazole as Inhibitor for C38 Steel Corrosion in 1.0 M H3PO4 Medium. J Fail. Anal. and Preven. 20, 563–571 (2020). https://doi.org/10.1007/s11668-020-00862-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-020-00862-5