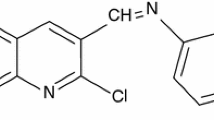
Abstract
The adsorption behavior and the inhibition performance of safranin, an azo dye, for mild steel corrosion in 0.5 M hydrochloric acid and citric acid solutions have been carried using potentiodynamic polarization and electrochemical impedance spectroscopy techniques as well quantum chemical calculations. Safranin has shown good inhibition efficiency in both acidic solutions. The impedance responses in both acids indicated that the corrosion process takes place under activation control. Langmuir and kinetic–thermodynamic adsorption isotherms were applied to clarify the mode of safranin inhibition. The obtained results revealed that the corrosion inhibition of mild steel in both acid solutions occurs through a physicochemical adsorption mechanism of safranin. The dissolution mechanism of mild steel in both acids was clarified and discussed.







Similar content being viewed by others
References
R.Y. Khaled, A.M. Abdel-Gaber, A comparative study of corrosion inhibition of steel and stainless steel in hydrochloric acid by N,N,N′,N′-tetramethyl-p-phenylenediamine. Prot. Met. Phys. Chem. Surf. 53(5), 956–960 (2017)
H.T. Rahal, A.M. Abdel-Gaber, G.O. Younes, Inhibition of steel corrosion in nitric acid by sulfur containing compounds. Chem. Eng. Commun. 203(4), 435–445 (2016)
K.M. Hijazi, A.M. Abdel-Gaber, G.O. Younes, Influence of malus domestica and Caesalpinia bonducella leaf extracts on the corrosion behaviour of mild steel in H2SO4 solution. Int. J. Electrochem. Sci. 10, 4779–4792 (2015)
I.R. Saad, A.M. Abdel-Gaber, G.O. Younes, B. Nsouli, Thiourea and N-methylthiourea as corrosion inhibitors for steel in phosphoric acid. JFAP 18(5), 1293–1299 (2018)
Y Avdeev, M Tyurina, V Rabinkov, A Luchkin, Inhibitive protection of low-carbon steel in citric acid solutions, in MATEC Web of Conferences, EDP Sciences, vol. 117, p. 00143 (2017)
T. Peme, L. Olasunkanmi, I. Bahadur, A. Adekunle, M. Kabanda, E. Ebenso, Adsorption and corrosion inhibition studies of some selected dyes as corrosion inhibitors for mild steel in acidic medium: gravimetric, electrochemical, quantum chemical studies and synergistic effect with iodide ions. Molecules 20(9), 16004–16029 (2015)
A.I. Onen, O.N. Maitera, J. Joseph, E.E. Ebenso, Corrosion inhibition potential and adsorption behaviour of bromophenol blue and thymol blue dyes on mild steel in acidic medium. Int. J. Electrochem. Sci. 6, 2884–2897 (2011)
V. Sivakumar, K. Velumani, S. Rameshkumar, Colocid dye-a potential corrosion inhibitor for the corrosion of mild steel in acid media. Mater. Res. 21(4), e20170167 (2018). https://doi.org/10.1590/1980-5373-MR-2017-0167
L. Tang, G. Mu, G. Liu, The effect of neutral red on the corrosion inhibition of cold rolled steel in 1.0 M hydrochloric acid. Corros. Sci. 45(10), 2251–2262 (2003)
E.E. Oguzie, Influence of halide ions on the inhibitive effect of Congo red dye on the corrosion of mild steel in sulphuric acid solution. Mater. Chem. Phys. 87(1), 212–217 (2004)
L. Tang, X. Li, G. Mu, L. Li, G. Liu, Synergistic effect between 4-(2-pyridylazo) resorcin and chloride ion on the corrosion of cold rolled steel in 1.0 M phosphoric acid. Appl. Surf. Sci. 253(18), 2367–2372 (2006)
V. Chandane, V.K. Singh, Adsorption of safranin dye from aqueous solutions using a low-cost agro-waste material soybean hull. Desalin. Water Treat. 57(9), 4122–4134 (2016)
J.D. Talati, N.H. Joshi, Corrosion of aluminium in aliphatic amines and its inhibition by some dyes. Mater. Corros. 31(12), 926–933 (1980)
A. Romeiro, C. Gouveia-Caridade, C.M. Brett, Polyphenazine films as inhibitors of copper corrosion. J. Electroanal. Chem. 688, 282–288 (2013)
E.E. Ebenso, E.E. Oguzie, Corrosion inhibition of mild steel in acidic media by some organic dyes. Mater. Lett. 59(17), 2163–2165 (2005)
S.A. Jebreil, Inhibition of mild steel corrosion in hydrochloric acid solutions by adsorption using safranin O dye, in ICCPGE, Libya (2016)
R.S. Al-Moghrabi, A.M. Abdel-Gaber, H.T. Rahal, A comparative study on the inhibitive effect of Crataegus oxyacantha and Prunus avium plant leaf extracts on the corrosion of mild steel in hydrochloric acid solution. IJIC 9(3), 255–263 (2018)
I. Mickova, A. Prusi, T. Grcev, L. Arsov, Electrochemical passivation of niobium in KOH solutions. Croat. Chem. Acta 79(4), 527–532 (2006)
A.M. Abdel-Gaber, E. Khamis, H. Abo-ElDahab, S. Adeel, Inhibition of aluminium corrosion in alkaline solutions using natural compound. Mater. Chem. Phys. 109(2–3), 297–305 (2008)
A. Diab, S.A. El-Haleem, Inhibition effect of some organic compounds toward the corrosion of steel electrode in citric acid solution infected by way of chloride ions. OJC 33(4), 1921–1932 (2017)
A.M. Abdel-Gaber, M.S. Masoud, E.A. Khalil, E.E. Shehata, Electrochemical study on the effect of Schiff base and its cobalt complex on the acid corrosion of steel. Corros. Sci. 51(12), 3021–3024 (2009)
I. Langmuir, The constitution and fundamental properties of solids and liquids. Part I. Solids. J. ACS 38(11), 2221–2295 (1916)
A.A. El-Awady, B.A. Abd-El-Nabey, S.G. Aziz, Kinetic-thermodynamic and adsorption isotherms analyses for the inhibition of the acid corrosion of steel by cyclic and open-chain amines. J. Electrochem. Soc. 139(8), 2149–2154 (1992)
R. Karthikaiselvi, S. Subhashini, Study of adsorption properties and inhibition of mild steel corrosion in hydrochloric acid media by water soluble composite poly(vinyl alcohol-omethoxy aniline). J. Assoc. Arab Univ. Basic Appl. 16(1), 74–82 (2014)
G. Lyberatos, L. Kobotiatis, Inhibition of aluminum 7075 alloy corrosion by the concerted action of nitrate and oxalate salts. Corrosion 47(11), 820–824 (1991)
A.M. Abdel-Gaber, B.A. Abd-El-Nabey, I.M. Sidahmed, A.M. El-Zayady, M. Saadawy, Inhibitive action of some plant extracts on the corrosion of steel in acidic media. Corros. Sci. 48(9), 2765–2779 (2006)
D.E. Abd-El-Khalek, A.M. Abdel-Gaber, Evaluation of nicotiana leaves extract as corrosion inhibitor for steel in acidic and neutral chloride solutions. Portugaliae Electrochim. Acta 30(4), 247–259 (2012)
A.M. Abdel-Gaber, N. Khalil, A. Abou El-Fetouh, The dissolution mechanism of steel in inorganic acids. Anti-Corros. Method M 50(6), 442–447 (2003)
H. Ju, Z.P. Kai, Y. Li, Aminic nitrogen-bearing polydentate Schiff base compounds as corrosion inhibitors for iron in acidic media: a quantum chemical calculation. Corros. Sci. 50(3), 865–871 (2008)
K.F. Khaled, Corrosion control of copper in nitric acid solutions using some amino acids—a combined experimental and theoretical study. Corros. Sci. 52(10), 3225–3234 (2010)
S. Xia, M. Qiu, L. Yu, F. Liu, H. Zhao, Molecular dynamics and density functional theory study on relationship between structure of imidazoline derivatives and inhibition performance. Corros. Sci. 50(7), 2021–2029 (2008)
A.Y. Musa, A.A.H. Kadhum, A.B. Mohamad, A.A.B. Rahoma, H. Mesmari, Electrochemical and quantum chemical calculations on 4,4-dimethyloxazolidine-2-thione as inhibitor for mild steel corrosion in hydrochloric acid. Mol. Struct. 969(1–3), 233–237 (2010)
I.B. Obot, E.E. Ebenso, I.A. Akpan, Z.M. Gasem, A.S. Afolabi, Thermodynamic and density functional theory investigation of sulphathiazole as green corrosion inhibitor at mild steel/hydrochloric acid interface. Int. J. Electrochem. Sci. 7, 1978–1996 (2012)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Sayed, M.Y., Abdel-Gaber, A.M. & Rahal, H.T. Safranin—A Potential Corrosion Inhibitor for Mild Steel in Acidic Media: A Combined Experimental and Theoretical Approach. J Fail. Anal. and Preven. 19, 1174–1180 (2019). https://doi.org/10.1007/s11668-019-00719-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-019-00719-6