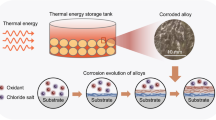
Abstract
The corrosion resistance of structural materials, particularly in molten salt environments, is of central importance to design concentrated solar power (CSP) plants. In this perspective, the high-temperature electrochemical behavior of passive film on 316SS in solar salt composition (60 pct. NaNO3: 40 pct. KNO3 by wt. pct.) was evaluated using linear resistance polarization, Tafel polarization, and electrochemical impedance spectroscopy techniques in the application range of 400 to 550 °C. An increase in corrosion rate with temperature and severe oxidation at 550 °C was recorded. However, the corrosion potential (Ecorr) does not vary significantly. The critical analysis of the impedance bode phase diagram reveals two well-separated maxima at 400 °C, indicating the role of the passive layer during the corrosion process. At 500 °C, the observed phase angle is close to 45°, attributed to processes controlled by mass transfer limitations. While analyzing the influence of mass transfer, an equivalent circuit model has been proposed to analyze the corrosion of the 316SS, a material used for piping and containment of CSP plants in molten solar salt.






Similar content being viewed by others
References
K. Vignarooban, X. Xu, A. Arvay, K. Hsu and A.M. Kannan, Heat Transfer Fluids for Concentrating Solar Power Systems – A Review, Appl. Energy, 2015, 146, p 383–396.
D. Kearney, B. Kelly, U. Herrmann, R. Cable, J. Pacheco, R. Mahoney, H. Price, D. Blake, P. Nava and N. Potrovitza, Engineering Aspects of a Molten Salt Heat Transfer Fluid in a Trough Solar Field, Energy, 2004, 29(5), p 861–870.
A.A. Attia, A.-H. Ali, A.N.A. Masri and A.M. Baraka, Corrosion Behaviour of Stainless Steel Alloys in Molten (Na, K)NO3 Eutectic Mixture, Mat.-wiss. u. Werkstofftech., 1999, 30(9), p 559–565.
A.G. Fernández, A. Rey, I. Lasanta, S. Mato, M.P. Brady and F.J. Pérez, Corrosion of Alumina-Forming Austenitic Steel in Molten Nitrate Salts by Gravimetric Analysis and Impedance Spectroscopy, Mater. Corros., 2014, 65(3), p 267–275.
A.S. Dorcheh, R.N. Durham and M.C. Galetz, High Temperature Corrosion in Molten Solar Salt: The Role of Chloride Impurities, Mater. Corros., 2017, 68(9), p 943–951.
S.H. Goods and R.W. Bradshaw, Corrosion of Stainless Steels and Carbon Steel by Molten Mixtures of Commercial Nitrate Salts, J. Mater. Eng.Perform., 2004, 13(1), p 78–87.
W.-J. Cheng, D.-J. Chen and C.-J. Wang, High-Temperature Corrosion of Cr–Mo Steel in Molten LiNO3–NaNO3–KNO3 Eutectic Salt for Thermal Energy Storage, Sol. Energy Mater. Sol. Cells, 2015, 132, p 563–569.
A. Baraka, A.I. Abdel-Rohman and A.A.E. Hosary, Corrosion of Mild Steel in Molten Sodium Nitrate-Potassium Nitrate Eutectic, Br. Corros. J., 1976, 11(1), p 44–46.
R.L. Brockenbrough and F.S. Merritt, Eds., "Structural Steel Designer's Handbook," 3rd ed, (New York), McGraw-Hill, 1999
T. AmaroVicente, L.A. Oliveira, E.O. Correa, R.P. Barbosa, V.B.P. Macanhan and N.G. de Alcântara, Stress Corrosion Cracking Behaviour of Dissimilar Welding of AISI 310S Austenitic Stainless Steel to 2304 Duplex Stainless Steel, Metals, Multidisciplinary Digital Publishing Instit., 2018, 8(3), p 195.
C.A.C. Sequeira, "High Temperature Corrosion in Molten Salts |," n.d., https://www.scientific.net/book/high-temperature-corrosion-in-molten-salts/978-3-0357-0603-1. Accessed 13 August 2021
J.W. Slusser, J.B. Titcomb, M.T. Heffelfinger and B.R. Dunbobbin, Corrosion in Molten Nitrate-Nitrite Salts, JOM, 1985, 37(7), p 24–27.
R.W. Bradshaw and S.H. Goods, Effect of Temperature on Corrosion of type 316S in molten nitrate salts, Canada, Toronto, 2000.
A. Kruizenga and D. Gill, Corrosion of Iron Stainless Steels in Molten Nitrate Salt, Energy Procedia, 2014, 49, p 878–887.
J.C. Gomez-Vidal, A.G. Fernandez, R. Tirawat, C. Turchi and W. Huddleston, Corrosion Resistance of Alumina-Forming Alloys against Molten Chlorides for Energy Production. I: Pre-Oxidation Treatment and Isothermal Corrosion Tests, Solar Energy Mater. Solar Cells, 2017, 166, p 222–233.
I.B. Singh, G. Venkatachari and K. Balakrishnan, Electrochemical Studies on the Oxidation Behaviour of Iron in NaNO3-NaNO2 Melt, Corros. Sci., 1994, 36(10), p 1777–1787.
Y.S. Cohen, Y. Gabay and Y. Cohen, Temperature-Dependent Impedance Spectroscopy of Molten Alkali-Halide Salt Binary Mixtures, ECS Electrochem. Lett., 2015, 4(1), p H1–H4.
Y. Grosu, U. Nithiyanantham, A. Zaki and A. Faik, A Simple Method for the Inhibition of the Corrosion of Carbon Steel by Molten Nitrate Salt for Thermal Storage in Concentrating Solar Power Applications, npj Mater. Degrad., 2018, 2(1), p 1–8.
G. García-Martín, M.I. Lasanta, V. Encinas-Sánchez, M.T. de Miguel and F.J. Pérez, Evaluation of Corrosion Resistance of A516 Steel in a Molten Nitrate Salt Mixture Using a Pilot Plant Facility for Application in CSP Plants, Sol. Energy Mater. Sol. Cells, 2017, 161, p 226–231.
A. Sandoval-Amador, A.J. Santander-Vega, C.C. Amaya-Cáceres, H.A. Estupiñán-Duran, and D.Y. Peña-Ballesteros, 316L Stainless Steel Corrosion in Molten Salts NaNO3 KNO3 NaNO2 Simulating Storage Conditions, J. Phys.: Conf. Ser., IOP Publishing, 2019, 1159, p 012011
Y. Grosu, O. Bondarchuk and A. Faik, The Effect of Humidity, Impurities and Initial State on the Corrosion of Carbon and Stainless Steels in Molten HitecXL Salt for CSP Application, Sol. Energy Mater. Sol. Cells, 2018, 174, p 34–41.
C.L. Zeng, W. Wang and W.T. Wu, Electrochemical Impedance Models for Molten Salt Corrosion, Corros. Sci., 2001, 43(4), p 787–801.
M. Wang, S. Zeng, H. Zhang, M. Zhu, C. Lei and B. Li, Corrosion Behaviors of 316 Stainless Steel and Inconel 625 Alloy in Chloride Molten Salts for Solar Energy Storage, High Temp. Mater. Processes De Gruyter, 2020, 39(1), p 340–350.
J.C. Gomez-Vidal, A.G. Fernandez, R. Tirawat, C. Turchi and W. Huddleston, Corrosion Resistance of Alumina Forming Alloys against Molten Chlorides for Energy Production. II: Electrochemical Impedance Spectroscopy under Thermal Cycling Conditions, Solar Energy Mater. Solar Cells, 2017, 166, p 234–245.
C.S. Ni, L.Y. Lu, C.L. Zeng and Y. Niu, Evaluation of Corrosion Resistance of Aluminium Coating with and without Annealing against Molten Carbonate Using Electrochemical Impedance Spectroscopy, J. Power Sour., 2014, 261, p 162–169.
J.C. Gomez-Vidal, Corrosion Resistance of MCrAlX Coatings in a Molten Chloride for Thermal Storage in Concentrating Solar Power Applications, npj Mater Degrad, 2017, 1(1), p 1–9.
A. Mallco and A.G. Fernández, Corrosion Monitoring Assessment on Lithium Nitrate Molten Salts as Thermal Energy Storage Material Applied to CSP Plants, Oxid. Met., 2020, 94(5), p 383–396.
C.L. Zeng and J. Li, Electrochemical Impedance Studies of Molten (0.9Na,0.1K)2SO4-Induced Hot Corrosion of the Ni-Based Superalloy M38G at 900°C in Air, Electrochimica Acta, 2005, 50(28), p 5533–5538.
M. Sarvghad, G. Will and T.A. Steinberg, Corrosion of Steel Alloys in Molten NaCl + Na2SO4 at 700°C for Thermal Energy Storage, Sol. Energy Mater. Sol. Cells, 2018, 179, p 207–216.
N.H. Abu-Hamdeh and K.A. Alnefaie, Design Considerations and Construction of an Experimental Prototype of Concentrating Solar Power Tower System in Saudi Arabia, Energy Convers. Manage., 2016, 117, p 63–73. https://doi.org/10.1016/j.enconman.2016.02.077
E. Mohammadi Zahrani and A.M. Alfantazi, Molten Salt Induced Corrosion of Inconel 625 Superalloy in PbSO4–Pb3O4–PbCl2–Fe2O3–ZnO Environment, Corrosion Sci., 2012, 65, p 340–359. https://doi.org/10.1016/j.corsci.2012.08.035
J.I. Barraza-Fierro, M.A. Espinosa-Medina, M. Hernandez-Hernandez, H.B. Liu and E. Sosa-Hernandez, Effect of Li and Cu Addition on Corrosion of Fe–40at.% Al Intermetallics in Molten LiCl–KCl Eutectic Salt, Corrosion Sci., 2012, 59, p 119–126. https://doi.org/10.1016/j.corsci.2012.02.020
E. Otero, A. Pardo, F.J. Perez, M.V. Utrilla and T. Levi, Corrosion Behavior of 12CrMoV Steel in Waste Incineration Environments: Hot Corrosion by Molten Chlorides, Oxid. Met., 1998, 49(5–6), p 467–484. https://doi.org/10.1023/A:1018851029023
BPL SG8 9AZ. (01763) 222 333 The Sty, 47 Upper King Street, Royston, Hertfordshire, "Platinum as a Reference Electrode in Electrochemical Measurements," Johnson Matthey Technology Review, n.d., https://www.technology.matthey.com/article/52/2/100-106/. Accessed 9 April 2018
E.M. Zahrani and A.M. Alfantazi, Corrosion Behavior of Alloy 625 in PbSO4-Pb3O4-PbCl2-ZnO-10 Wt Pct CdO Molten Salt Medium, Metall. Mat. Trans. A, 2012, 43(8), p 2857–2868. https://doi.org/10.1007/s11661-011-0996-1
"ASTM G102 - 89(2015)E1 Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements," n.d., https://www.astm.org/Standards/G102.htm. Accessed 25 June 2017
“SAND2013-2526 - 132526.Pdf,” n.d., http://prod.sandia.gov/techlib/access-control.cgi/2013/132526.pdf. Accessed 22 June 2017
J.R. Macdonald, Impedance Spectroscopy: Old Problems and New Developments, Electrochim. Acta, 1990, 35(10), p 1483–1492.
F. Mansfeld, Electrochemical Impedance Spectroscopy (EIS) as a New Tool for Investigating Methods of Corrosion Protection, Electrochim. Acta, 1990, 35(10), p 1533–1544.
G. Gao, F.H. Stott, J.L. Dawson and D.M. Farrell, Electrochemical Monitoring of High-Temperature Molten-Salt Corrosion, Oxid. Met., 1990, 33(1–2), p 79–94.
Acknowledgment
Authors would like to acknowledge the Solar Energy Research Initiative (SERI) -Department of Science and Technology (DST) for their financial support. MPS wants to thank Punith Kumar M K for familiarizing him with several corrosion testing techniques.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, M.P., Basu, B. & Chattopadhyay, K. Probing High-Temperature Electrochemical Corrosion of 316 Stainless Steel in Molten Nitrate Salt for Concentrated Solar Power Plants. J. of Materi Eng and Perform 31, 4902–4908 (2022). https://doi.org/10.1007/s11665-021-06538-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-021-06538-x