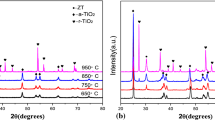
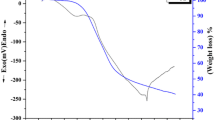
In this study, thin-film coating of zirconium oxide (ZrO2) was prepared by sol-gel method and subsequent heat treatment process. The sol was prepared by controlled hydrolysis of zirconium tetrapropoxide using acetic acid and ethanol/acetylacetone mixture as catalyst and chelating agent, respectively, and finally deposited onto the 316L austenitic stainless steel (316L SS) using dip coating method in order to improve its corrosion resistance in nitric acid medium. The composition, structure, and morphology of the coated surface were investigated by x-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and atomic force microscopy (AFM). The obtained results from XRD and FTIR state the formation of tetragonal and monoclinic ZrO2 phase. Also, the obtained results from surface morphology investigation by SEM and AFM indicate the formation of smooth, homogeneous and uniform coatings on the steel substrate. Then, the corrosion behavior of stainless steel was investigated in a 1 and 10 M nitric acid solutions using electrochemical impedance spectroscopy and linear polarization test. The obtained results from these tests for ZrO2-coated specimens indicated a considerable improvement in the corrosion resistance of 316L stainless steel by an increase in corrosion potential and transpassive potential, and a decrease in passive current density and corrosion current density. The decrease in passive current density in both the concentration of solutions was two orders of magnitude from bare to coated specimens.










Similar content being viewed by others
References
S. Yang and D.D. Macdonald, Theoretical and Experimental Studies of the Pitting of Type 316L Stainless Steel in Borate Buffer Solution Containing Nitrate Ion, Electrochim. Acta, 2007, 52(5), p 1871–1879
K. Ramana, T. Anita, S. Mandal, S. Kaliappan, H. Shaikh, P. Sivaprasad et al., Effect of Different Environmental Parameters on Pitting Behavior of AISI, type 316L Stainless Steel: Experimental Studies and Neural Network Modeling, Mater. Des., 2009, 30(9), p 3770–3775
U.K. Mudali, R. Dayal, and J. Gnanamoorthy, Corrosion Studies on Materials of Construction for Spent Nuclear Fuel Reprocessing Plant Equipment, J. Nucl. Mater., 1993, 203(1), p 73–82
S. Haupt and H.-H. Strehblow, A Combined Surface Analytical and Electrochemical Study of the Formation of Passive Layers on FeCr Alloys in 0.5 M H2SO4, Corros. Sci., 1995, 37(1), p 43–54
R. Robin, F. Miserque, and V. Spagnol, Correlation Between Composition of Passive Layer and Corrosion Behavior of High Si-Containing Austenitic Stainless Steels in Nitric Acid, J. Nucl. Mater., 2008, 375(1), p 65–71
V. Bague, S. Chachoua, Q. Tran, and P. Fauvet, Determination of the Long-Term Intergranular Corrosion Rate of Stainless Steel in Concentrated Nitric Acid, J. Nucl. Mater., 2009, 392(3), p 396–404
M. Mayuzumi, J. Ohta, and K. Kako, Corrosion Behavior of High-Purity Fe-Cr-Ni Alloys in the Transpassive Condition, Corrosion., 2000, 56(1), p 70–79
A. Desestret, I. Epelboin, M. Froment, and P. Guiraldenq, Sur la comparaison des facies D’attaque electrolytique et thermique D’aciers inoxydables austenitiques a teneur variable En Si, Corros. Sci., 1968; 8(4):225IN9229–228IN12234
A. Balamurugan, S. Rajeswari, G. Balossier, A. Rebelo, and J. Ferreira, Corrosion Aspects of Metallic Implants—An Overview, Mater. Corros., 2008, 59(11), p 855–869
C. Bayram, A.K. Mizrak, S. Aktürk, H. Kurşaklioğlu, A. Iyisoy, A. Ifran et al., In Vitro Biocompatibility of Plasma-Aided Surface-Modified 316L Stainless Steel for Intracoronary Stents, Biomed. Mater., 2010, 5(5), p 055007
Y. Dianran, H. Jining, W. Jianjun, Q. Wanqi, and M. Jing, The Corrosion Behaviour of a Plasma Spraying Al2O3 Ceramic Coating in Dilute HC1 Solution, Surf. Coat. Technol., 1997, 89(1–2), p 191–195
K. Ramachandran, V. Selvarajan, P. Ananthapadmanabhan, and K. Sreekumar, Microstructure, Adhesion, Microhardness, Abrasive Wear Resistance and Electrical Resistivity of the Plasma Sprayed Alumina and Alumina–Titania Coatings, Thin Solid Films, 1998, 315(1), p 144–152
A. Afrasiabi, M. Saremi, and A. Kobayashi, A Comparative Study on Hot Corrosion Resistance of Three Types of Thermal Barrier Coatings: YSZ, YSZ + Al 2 O 3 and YSZ/Al 2 O 3, Mater. Sci. Eng. A, 2008, 478(1), p 264–269
K. Meinert, C. Uerpmann, J. Matschullat, and G. Wolf, Corrosion and Leaching of Silver Doped Ceramic IBAD Coatings on SS 316L Under Simulated Physiological Conditions, Surf. Coat. Technol., 1998, 103, p 58–65
Z. Zhou, E. Chalkova, S. Lvov, and P. Chou, Hydrothermal Deposition of Zirconia Coatings on Pre-oxidized BWR Structural Materials, J. Nucl. Mater., 2008, 378(3), p 229–237
K. Rodrigo, J. Knudsen, N. Pryds, J. Schou, and S. Linderoth, Characterization of Yttria-Stabilized Zirconia Thin Films Grown by Pulsed Laser Deposition (PLD) on Various Substrates, Appl. Surf. Sci., 2007, 254(4), p 1338–1342
S. Ushakov, A. Navrotsky, Y. Yang, S. Stemmer, K. Kukli, M. Ritala et al., Crystallization in Hafnia-and Zirconia-Based Systems, Physica status solidi, 2004, 241(10), p 2268–2278
M. Putkonen and L. Niinistö, Zirconia Thin Films by Atomic Layer Epitaxy. A Comparative Study on the uSe of Novel Precursors with Ozone, J. Mater. Chem., 2001, 11(12), p 3141–3147
C. Guizard, N. Cygankiewicz, A. Larbot, and L. Cot, Sol-Gel Transition in Zirconia Systems Using Physical and Chemical Processes, J. Non-Cryst. Solids, 1986, 82(1–3), p 86–91
D.A. Ward and E.I. Ko, Use Of Preformed Sols in The Sol-Gel Preparation of Zirconia, Langmuir, 1995, 11(1), p 369–372
T.A. Kuriakose, S.N. Kalkura, M. Palanichamy, D. Arivuoli, K. Dierks, G. Bocelli et al., Synthesis of Stoichiometric Nano Crystalline Hydroxyapatite by Ethanol-Based Sol–Gel Technique at Low Temperature, J. Cryst. Growth, 2004, 263(1), p 517–523
C.J. Brinker and G.W. Scherer, Sol-Gel Science, Academic Press, San Diego, 1990, p 2
M. Norouzia and A.A. Garekani, Corrosion Protection by Zirconia-Based Thin Films Deposited by a Sol–Gel Spin Coating Method, Ceram. Int., 2014, 40(2), p 2857–2861
I. Bačić, H.O. Ćurković, L. Ćurković, V. Mandić, and Z. Šokčević, Corrosion Protection of AISI, 316L Stainless Steel with the Sol-Gel Yttria Stabilized ZrO2 Films: Effects of Sintering Temperature and Doping, Int. J. Electrochem. Sci., 2016, 11(11), p 9192–9205
A. Bu, J. Wang, J. Zhang, J. Bai, Z. Shi, Q. Liu, and G. Ji, Corrosion Behavior of ZrO2–TiO2 Nanocomposite Thin Films Coating on Stainless Steel Through Sol–Gel Method, J. Sol-Gel. Sci. Technol., 2017, 81(3), p 633–638
S.J. Kang and H.T. Kima, Sol–gel Synthesis of Photoactive Zirconia–Titania from Metal Salts and Investigation of Their Photocatalytic Properties in the Photodegradation of Methylene Blue, Powder Technol., 2014, 258(8), p 99–109
D. Pamu, K. Sudheendran, M.G. Krishna, and K.C.J. Raju, Dielectric Properties of Ambient Temperature Grown Nanocrystalline ZrTiO4 Thin Films Using DC Magnetron Sputtering, Mater. Sci. Eng., B, 2010, 168(1), p 208–213
A. Huang and P.K. Chu, Microstructural Improvement of Sputtered ZrO 2 Thin Films by Substrate Biasing, Mater. Sci. Eng., B, 2005, 121(3), p 244–247
G. Štefanić, S. Popović, and S. Musić, Influence of Cr 2 O 3 on the Stability of Low Temperature t-ZrO 2, Mater. Lett., 1998, 36(5), p 240–244
S. Tiwari, J. Adhikary, T. Singh, and R. Singh, Preparation and Characterization of Sol–Gel Derived Yttria Doped Zirconia Coatings on AISI, 316L, Thin Solid Films, 2009, 517(16), p 4502–4508
H. Wang, G. Li, Y. Xue, and L. Li, Hydrated Surface Structure and its Impacts on the Stabilization of t-ZrO 2, J. Solid State Chem., 2007, 180(10), p 2790–2797
K. Izumi, M. Murakami, T. Deguchi, A. Morita, N. Tohge, and T. Minami, Zirconia Coating on Stainless Steel Sheets from Organozirconium Compounds, J. Am. Ceram. Soc., 1989, 72(8), p 1465–1468
P. Pouilleau, D. Devillier, F. Garrido, S. Durand-Vidal, and E. Mah, Structure and Composition of Passive Titanium Oxide Films, Mater. Sci. Eng., B, 1997, 47, p 235–243
C. Liu, Q. Bi, A. Leyland, and A. Matthews, An Electrochemical Impedance Spectroscopy Study of the Corrosion Behaviour of PVD Coated Steels in 0.5 N NaCl Aqueous Solution: Part II.: EIS Interpretation of Corrosion Behavior, Corros. Sci., 2003, 45(6), p 1257–1273
A.R. Shankar and U.K. Mudali, Refractory Metal Coatings on Titanium to Improve Corrosion Resistance in Nitric Acid Medium, Surf. Coat. Technol., 2013, 235(1), p 155–164
A. Takarada, T. Suzuki, K. Kanda, M. Niibe, M. Nakanod, N. Ohtake, and H. Akasaka, Structural Dependence of Corrosion Resistance of Amorphous Carbon Films Against Nitric Acid, Diam. Relat. Mater., 2015, 51(1), p 49–54
S. Ningshen, U.K. Mudali, R. Krishnan, and B. Raj, Corrosion Behavior of Zr-Based Metallic Glass Coating on Type 304L Stainless Steel by Pulsed Laser Deposition Method, Surf. Coat. Technol., 2011, 205(15), p 3961–3966
N. Padhy, S. Kamal, R. Chandra, U.K. Mudali, and B. Raj, Corrosion Performance of TiO2 Coated Type 304L Stainless Steel in Nitric Acid Medium, Surf. Coat. Technol., 2010, 204(16), p 2782–2788
Acknowledgment
The authors wish to gratefully acknowledge Mrs. Saremi for her assistance in the electrochemical laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kazazi, M., Haghighi, M., Yarali, D. et al. Improving Corrosion Resistance of 316L Austenitic Stainless Steel Using ZrO2 Sol-Gel Coating in Nitric Acid Solution. J. of Materi Eng and Perform 27, 1093–1102 (2018). https://doi.org/10.1007/s11665-018-3202-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-018-3202-4