Abstract
The low-alloy steel was nitrided in a pure NH3 gas atmosphere at 640 ~ 660 °C for 2 h, i.e., high-temperature gas nitriding (HTGN), followed by tempering at 225 °C, which can produce a high property surface coating without brittle compound (white) layer. The steel was also plasma nitriding for comparison. The composition, microstructure and microhardness of the nitrided and tempered specimens were examined, and their tribological behavior investigated. The results showed that the as-gas-nitrided layer consisted of a white layer composed of FeN0.095 phase (nitrided austenite) and a diffusional zone underneath the white layer. After tempering, the white layer was decomposed to a nano-sized (α-Fe + γ′-Fe4N + retained austenite) bainitic microstructure with a high hardness of 1150HV/25 g. Wear test results showed that the wear resistance and wear coefficient yielded by the complex HTGN plus tempering were considerably higher and lower, respectively, than those produced by the conventional plasma nitriding.
Similar content being viewed by others
Introduction
The traditional nitriding is usually carried out below the Fe-N eutectoid temperature of 590 °C, which is widely used for steel components in order to improve their wear, corrosion and fatigue properties by forming a hard, multiphase compound layer and a tough diffusion layer (Ref 1,2,3). However, this compound, or white layer, consisting of a heterogeneous mixture of γ′-Fe4N and ε-Fe2-3N phases, contains high internal stresses in the transitional regions between the various lattice structures (Ref 4). Although this layer has a relatively high hardness of 950-1200 HV, the internal stresses make it friable and brittle. The compound layer spalls during service, when the components subjected to severe service environments involving high shear, compressive and/or impact loading conditions (Ref 5,6,7,8,9). So, measures must be taken to avoid forming the brittle compound layer during nitriding, such as optimizing the nitriding procedure to obtain a single γ′-Fe4N phase and decomposing the compound layer after nitriding to form a nitride layer without compound layer (Ref 10, 11). However, these measures will induce deterioration of the surface hardness and wear performance of the nitrided steel.
A technique was then developed in which gas nitriding was performed on pure iron sheet at 640 °C, a temperature higher than the conventional nitriding temperature, to obtain a nitrided austenite which can be retained at room temperature, and was then tempered. The nitrided austenite formed during nitriding can be retained at room temperature because a) the martensite start temperature is lowered considerably by the nitrogen, and b) the quenching rate was fast enough to suppress its thermally activated decomposition during cooling. It was found that the hardness of the 225 °C tempered product of the nitrided austenite reached 1000 HV with a load of 25 g (Ref 12). Further researches, conducted by the authors, showed that the transformation products consisted of nano-sized α-Fe, γ′-Fe4N and retained austenite. The transformation was thought to be a bainitic one in nature (Ref 13,14,15). Wang’s et al. (Ref 16) investigations also show that the forming of α’-Fe and γ’-Fe4N phases in the surface layer by plasma nitriding can enhance wear resistance significantly. Based on the above research results, it is reasonable to believe that a high-temperature gas nitriding (HTGN) plus tempering can improve the surface property of low-alloy steel. Therefore, a further study in this direction with a commercial steel 20 Cr seems to be necessary for commercial applications.
The purpose of this work is thus to study the structural, tribological property of the transformation products formed on 20 Cr steel subjected to the HTGN plus temper treatments. The microstructural, mechanical and tribological performances of the nitrided layer were characterized with XRD, scanning electron microscopy (SEM), microhardness tester and ball-on-disk tribotester. For comparison, similar properties of plasma-nitrided 20 Cr were also investigated.
Experimental
Specimens, φ30 × 6 mm and 10 × 10 × 3 mm in size, of a 20 Cr steel containing (wt.%): 0.23 C, 0.52 Si, 0.55 Mn, 1.07 Cr, 0.035 P (max), 0.035 S(max) and balance Fe, were nitrided in an ammonia gas at 640-660 °C for 2 h, at an ammonia dissociation rate of about 98%, and a flow rate of 0.24 mL/s, and then oil-quenched. Prior to the gas nitriding treatment, the specimens were austenitized at 880 °C for 20 min, water-quenched and tempered at 650 °C for 2 h, which are a conventional heat treatment for the 20 Cr steel. The gas nitriding apparatus used was a laboratory-made horizontal quartz tube furnace, the gas flow was controlled by a mass flow controller, and the ammonia dissociation controlled by a hydrogen analyzer. The nitrided specimens were then tempered in molten nitrate bath (53%KNO3 + 40%NaNO2 + 7%NaNO3) at 225(± 2) °C, 255(± 2) °C and 300(± 2) °C, respectively, for various durations. Meanwhile, 20 Cr specimens for comparison with the HTGN plus tempered ones were plasma-nitrided at 540-550 °C for 8 h, which is a conventional plasma nitriding for low-alloy steel.
Friction and wear tests were carried out on a standard ball-on-disk MS-T3000 tribometer, in which the disk-like specimen 30 mm in diameter was fixed on the vertical rotation axis. Bearing steel balls corresponding to GCr15 with a diameter of 6 mm were used as a counterpart, and the balls were quenched and tempered to a hardness of 59 ~ 61HRC. At the same time, block-on-ring test was also taken on M-2000 tribometer, in which the block specimen 10 × 10 × 3 mm was fixed on the rotating ring periphery (GCr15 steel, 59-61HRC, φ40 × 10 mm) under various loadings. Wear tests were performed at a rotating speed of 214 rpm at various loads. This testing was conducted in an ambient atmosphere at a temperature around 25 °C and a relative humidity of 75% without lubrication.
The microstructure of the treated surface layer was characterized by a Leica DMR optical microscope (OM), and both Hitachi M3700 and Nova NanoSEM 430 scanning electron microscopes (SEM). A HV1000 Vickers microhardness tester was used to determine the surface hardness and the variation of hardness with distance from the surface. For each of the hardness, five readings were measured from randomly chosen regions and used to calculate the averaged values of hardness, and the standard deviations were also calculated. The phases in the surface layer were identified by means of Philip X’Pert Pro x-ray diffractometer (XRD) using Cu Kα radiation (40 kV, 40 mA).
Results and Discussion
Figure 1 shows the cross-sectional micrographs of the HTGN plus oil-quenched, as well as the plasma-nitrided 20 Cr steel specimens. Obviously, the nitrided layers of the two specimens all contain a surface “white layer” and a diffusion zone. Figure 1(a) and (b) shows that the average thickness of the “white layer” is about 30 μm for the HTGN specimen and 8 μm for the plasma-nitrided one. XRD indicates that the “white layer” mainly consisted of γ-FeN0.0950 phase (i.e., nitrided austenite, JCPDS: 75-2136) for the HTGN specimen (Fig. 1c) and of a single ε-Fe24N10 phase (JCPDS: 73-2103) for the plasma nitride one (Fig. 1d). Some researchers may think that is well to confirm the formation of high nitrogen austnite at the ambient temperature by measuring the nitrogen concentration in austenite, and then calculations of Ms temperature. The Ms temperature should be lower than ambient temperature, and the cooling rate is quick enough to avoid the phase transformation during cooling process. It is complex. The formation of high nitrogen austenite after nitriding was confirmed directly by XRD analysis in this research. The CrN or Cr2N phase cannot be detected in the “white layer” for the two kinds of specimens, although there exists about 1% (wt.) Cr in the steel substrate. However, researches by Jessner (Ref 17) and Steiner (Ref 18) show chromium nitride precipitation on a nanoscale during nitriding, even in the nitriding temperature slightly lower than this work, and the decomposition temperature of chromium nitride is over 700 °C according to Cr-N binary phase diagram. So, there may be some chromium nitride precipitated after HTGN and tempering in this work.
(a) Cross-sectional optical microstructure of 20 Cr steel HTGN at 640-660 °C for 2 h and then oil-quenched, showing the “white layer” at the outer surface, and (c) it’s XRD spectra showing a single γ-FeN0.0950 phase existing in the layer; (b) cross-sectional optical microstructure of 20 Cr steel plasma-nitrided at 540 ~ 550 °C for 8 h, and (d) the corresponding XRD spectra showing a single ε-Fe24N10 phase in it
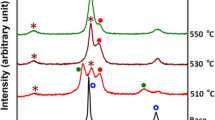
Figure 2 indicates the cross-sectional microstructure varying with tempering time of 0 h, 6 h, and 8 h at 225 °C for the HTGN plus tempered specimens. It is seen that with increase in the tempering time, more and more transformation product, composed of ultra-fine or even nano-sized crystals, is formed by decomposition of the nitrided austenite. As shown in Fig. 3, γ′-Fe4N (JCPDS: 77-2006) and α-Fe phases (JCPDS: 06-0696) are detected in the decomposition product.
Figure 3 illustrates the phase structure evolution of the HTGN “white layer” with tempering time at 225 °C. It is clear from Fig. 3 that the intensity of γ-FeN0.0950 phase diffraction is decreasing while that of the γ′-Fe4N and α-Fe is increasing with increase in the tempering time, in agreement with what is presented in Fig. 2, i.e., the decomposition of the “white layer” (γ-FeN0.0950) is increased with prolonging the tempering time. Decomposition of γ-FeN0.0950 phase will be ended after 8 h tempering at 225 °C, as shown in Fig. 2(c) and (d), which finally results in a nano-crystalline structure within the original “white layer.” This observation is in agreement with that reported in previous work (Ref 19).
Figure 4(a), (b), and (c) shows the microhardness profile of cross-sectional specimens tempered at different temperature (225, 255, and 300 °C) for various times. The peak hardness of the tempered specimens reached 1150HV/25 g by tempering at 225 °C for 8 h, at a position about 23 μm from the surface, and the peak hardness at two other temper temperatures is lower than that at 225 °C. With increase in the tempering temperature, the peak hardness decreases and the “incubation period” for reaching the peak hardness is gradually shortened. Obviously, surface hardening occurred due to the transformation product composed of nano-sized γ′-Fe4N, α-Fe and trace retained austenite. As reported earlier (Ref 15), a bainitic transformation might have taken place during tempering the γ-FeN0.0950 austenite at 225 °C, which produces a strong and tough microstructure. In addition, why we thought the transformation to be a bainitic one is because (a) the hardness of a perlitic transformation product is lower than that of the bainitic one; and (b) although the hardness of a martensitic transformation product can meet to the same level of the bainitic one in this work, the martensitic transformation product obtained by cooling with liquid nitrogen is morphologically different from that observed in this research, as shown in Fig. 5 (Ref 13 in Fig. 2). Because of the similarity of pure iron with the low carbon steel, it is reasonable to think that the transformation in this research is a bainitic one.
SEM/BEI (backscattered electron image) of high-N austenite cooled by liquid nitrogen and then aged at 225°C for 4 h (Ref 13)
The high hardness of the nano-sized bainitic microstructure could offer a superior tribological performance, particularly, an excellent abrasive wear resistance (Ref 20). The peak hardness obtained from the nitrided and tempered 20 Cr steel exceeded that from the pure iron subjected to the same treatments, possibly due to the formation of the CrN/Cr2N in 20 Cr steel, although they were not detected by XRD in the present study.
In order to investigate the wear resistance of the HTGN plus tempered specimens, rolling friction wear tests were conducted. Three groups of specimens, namely that tempered at 225 °C for 8 h, that tempered at 255 °C for 6 h, and that plasma-nitrided (nitrided at 540 ~ 550 °C for 8 h and surface hardness 900HV), were selected for the tests. Figure 6 shows friction curves for the HTGN plus tempered and plasma-nitrided specimens. The friction coefficients of both the HTGN plus tempered specimens at 225 °C for 8 h and 255 °C for 6 h have the similar overall profile, but the friction coefficient of 225 °C tempered specimen is lower than that of 255 °C one at the steady stage of friction. With continued rolling, the friction coefficient slightly decreases and tends to reach a steady state, in which a 0.38 for the 225 °C/8 h specimen and 0.44 for the 255 °C/6 h one are attained. However, the friction coefficient of plasma-nitrided specimen reaches 0.75, which indicates that the HTGN plus tempered specimens show a much better (lower) friction coefficient than the plasma-nitrided one, due to the hard surface of the former specimens, resulting from the nano-crystalline γ′-Fe4N, α-Fe and retained austenite formed in them (Ref 15). The decreased contact area on the hard surface would lead to a lower possibility of forming welded areas, which can avoid adhesive wear (Ref 21).
Figure 7 shows the wear volume of the specimens after 20 min block-on-ring wear test under the loads of 100, 150, and 200 N with 214 rad/s, respectively. The wear volumes were calculated according to the wear scratch on the specimens by integral:
ΔV is the wear volume of the specimen; r the radius of counterpart; h the width of counterpart; l 1 and l 2 the wear scratch width at the two ends of the specimen; and θ the counterpart center angle of chord l. Each wear scratch width was obtained by averaging three measurements, and the wear volume and their standard deviation were calculated based on it. It is clear that the HTGN plus tempered specimen at 225 °C/8 h achieves good tribological property.
To evaluate the tribological behavior and wear mechanism involved, the worn surfaces of the HTGN plus tempered (Fig. 8a) and the plasma-nitrided (Fig. 8b) specimens were examined by SEM. For the plasma nitride one, severe spalling occurred at the rolling contact zone with oxide debris adhering to it (Fig. 8b). The spalling is attributed to the fatigue wear caused by the fatigue cracks formed between the thin hardened layer and the substrate. The worn surface of the HTGN plus tempered (at 225 °C for 8 h) specimen is, however, characteristic of a smooth and metallic adhesion without obvious oxide debris piled up at the edge of the scratch grooves, and its wear is featured of plow friction, abrasive scratch and slight adhesive craters, as indicated by the shallow scratch grooves (Fig. 8a and c). EDS analysis performed in the rectangle area in Fig. 8(c) demonstrated that the worn products for the HTGN plus tempered surface were composed of (at.%): 70.58 Fe, 23.18 O, 5.52 N, 0.72 Cr, indicating the existence of oxidation wear.
As aforementioned, in a previous study (Ref 13) a high nitrogen austenite was produced by nitriding a pure iron sheet about 0.1 mm in thickness at a high temperature of about 640 °C (a temperature higher than the conventional nitriding temperature of 480-560 °C) and at a high ammonia dissociation rate of 98%. The nitrided austenite so obtained was then tempered at 225 °C, which generated a bainitic microstructure composed of nano-sized α-Fe, γ′-Fe4N and retained austenite with a high hardness of 1000HV.
In contrast to the thin pure iron sheet for which a through nitriding is possible and the nitriding process is simple because of the absence of any alloying elements in the material, a nitrided layer composed of single nitrided austenite similar to that obtained by nitriding the thin pure iron sheet has been successfully produced on a bulk steel 20 Cr by the same nitriding process (Fig. 3), and the nitrided austenite layer was then tempered at the same temperature of 225 °C to produce a hard and wear-resistant bainitic microstructure (nano-sized α-Fe + γ′-Fe4N + retained austenite). Zhang et al. (Ref 22) also reported that the nitrogen content in the nitrided layer was increased to 1.2 wt.%, martensitic transformation depressed, and nano-crystalline bainite formed in the layer by austempering, but its hardness was only 750HV, considerably lower than that of the transformation product in this research the higher hardness in this work is due to a higher phase fraction of hard particles and higher nucleation rate of the austenite phase at room temperature, which will form a finer particles size than that of N austenite with 1.2 wt.%N in Ref 22.
It is evident that the surface hardening, strengthening and toughening of the HTGN plus tempered steel can be attributed to the formation of the (nano-sized α-Fe + γ′-Fe4N + retained austenite) bainitic microstructure. The ultra-fine, even nano-sized (α-Fe + γ′-Fe4N) would be responsible for the surface hardening and toughening via precipitation and fine grain strengthening, which would retard the formation of worn grooves on the rolling contact surface. In addition, the low tempering temperature employed would not seriously deteriorate the strength of the diffusional zone/substrate, thus keeping a good adhesion and load-bearing capacity between the surface layer and the diffusional zone/substrate, and hence preventing surface spalling. This important finding of forming an ultra-fine bainitic microstructure by HTGN plus tempering would throw a light on improving the tribological performance of plain low-alloy steels such as 20 Cr; and this novel surface hardening technique would be of growing interest in surface protection and modification of steel components used in tribological applications. Meanwhile, the retained austenite might also to some extent be work-hardened or transformed to martensite under the wear-induced high pressure, thus further improving the wear resistance.
Conclusions
The high-temperature gas nitriding (HTGN) plus tempering was performed on a bulk 20 Cr steel, and a “white layer,” 30 μm in thickness and consisting of single γ-FeN0.0950 (nitrided austenite), was well formed within the nitrided surface layer. Subsequent tempering of the “white layer” at 225 °C for 8 h made the nitrided austenite decompose to an ultra-fine or even nano-sized (α-Fe + γ′-Fe4N + retained austenite) bainite with a high hardness 1150HV. The surface hardness and tribological properties of the steel were increased due to the strengthening and toughening brought about by the bainitic microstructure. A friction coefficient of 0.38 was attained, and an abrasive and/or oxidative wear mechanism found for the HTGN plus tempered specimens, in contrast to that of 0.75 and a fatigue wear mechanism for the plasma-nitrided specimens. This surface strengthening technique would throw a light on improving the tribological performance of plain low-alloy steels such as 20 Cr steel.
References
W.P. Tong, C.Z. Liu, W. Wang, N.R. Tao, Z.B. Wang, L. Zuo, and J.C. He, Gaseous Nitriding of Iron with a Nanostructured Surface Layer, Scr. Mater., 2007, 57, p 533–536
J. Mongis, J.P. Peyre, and C. Tournier, Nitriding of Microalloyed Steels, Heat Treat. Met., 1984, 3, p 71–75
T. Steiner and E.J. Mittemeijer, Alloy Element Nitride Development in Ferritic Fe-Based Materials Upon Nitriding: A Review, J. Mater. Eng. Perform., 2016, 25, p 2091–2102
J.A. Kirk, G.W. Egerton, and B.D. Sartwell, Wear and Friction of Nitrogen Ion Implanted Steel, J. Lubr. Technol., 1983, 105(2), p 239–244
A. Cohen and A. Rosen, The Influence of the Nitriding Process on the Dry Wear Resistance of 15-5PH Stainless Steel, Wear, 1986, 108, p 157–168
A. Celik and S. Karadeniz, Investigation of Compound Layer Formed During Ion Nitriding of AISI, 4140 Steel, Surf. Coat. Technol., 1996, 80, p 283–286
C. Kwietniewski, W. Fontana, C. Moraes, A.D.S. Rocha, T. Hirsch, and A. Regaly, Nitrided Layer Embrittlement Due to Edge Effect on Duplex Treated AISI, M2 High-Speed Steel, Surf. Coat. Technol., 2004, 179, p 27–32
D. Nolan, V. Leskovsek, and M. Jenko, Estimation of Fracture Toughness of Nitride Compound Layers on Tool Steel by Application of the Vickers Indentation Method, Surf. Coat. Technol., 2006, 201, p 182–188
T. Doggart and H. Gupta, An Investigation of Case-Hardened Nitralloy, J. Mater. Sci., 1997, 32, p 2059–2062
M. Keddam, B. Bouarour, R. Kouba, and R. Chegroune, Growth Kinetics of the Compound Layers: Effect of the Nitriding Potential, Phys. Proc., 2009, 2, p 1399–1403
S. Srikanth, P. Saravanan, A. Joseph, K. Ravl, Surface Modification of Commercial Low-Carbon Steel using Glow Discharge Nitrogen Plasma and its Characterization, J. Mater. Eng. Perform. 2013, 22, p 2610–2622
M.J. Hu, J.S. Pan, Z.C. Zhu, and C.C. Qiu, A Special Phase Transformation Phenomenon in High-Nitrogen Austenite, Mater. Lett., 2001, 50, p 225–229
D.L. Jiao, C.P. Luo, and J.W. Liu, Isothermal Transformation of High-Nitrogen Austenite, Scr. Mater., 2007, 56, p 613–616
D.L. Jiao, C.P. Luo, and J.W. Liu, Grain Boundary Transformation Character in Fe-N Austenite, Mater. Lett., 2012, 66, p 147–149
D.L. Jiao, C.P. Luo, and J.W. Liu, Morphology and Crystallographic Orientation Relationship in Isothermally Transformed Fe-N Austenite, Mater. Char., 2014, 88, p 52–57
X.A. Wang, M.F. Yan, C.S. Zhang, and Y.X. Zhang, Microstructure and Mechanical Properties of Surface Layer of M50NiL Steel Plasma Nitride, Surf. Eng., 2014, 30(3), p 218–223
P. Jessner, R. Danoix, B. Hannoyer, and F. Danoix, Investigations of the Nitrided Subsurface Layers of an Fe-Cr Model Alloy, Ultramicroscopy, 2009, 109, p 530–534
T. Steiner, S.R. Meka, B. Rheingans, E. Bischoff, T. Waldenmaier, G. Yeli, T.L. Martin, Paul A.J. Bagot, M.P. Moody, and E.J. Mittemeijer, Continuous and Discontinuous Precipitation in Fe-1 at.%Cr-1 at.%Mo Alloy Upon Nitriding; Crystal Structure and Composition of Ternary Nitrides, Phil. Mag., 2016, 96, p 1509–1537
D.L. Jiao, C.L. Luo, and J.W. Liu, Intermediate Temperature Transformation of High Nitrogen Austenite, Acta. Metall. Sin., 2007, 3, p 337–343
H. Kato, T.S. Eyre, and B. Ralph, Sliding Wear Characteristics of Nitrided Steels, Surf. Eng., 1994, 10, p 65–74
B.Y. Wang, Z.B. Hou, W. Wang, and B. Zhao, Investigation of Gas Nitriding on Wear and Corrosion Behavior of 40 Cr Steel, Adv. Mater. Res., 2011, 2011(311–313), p 674–678
P. Zhang, F.C. Zhang, Z.G. Yan, and C.L. Zheng, N Rich Nanocrystalline Bainite in Surface Layer of Carbon Steel, Surf. Eng., 2013, 29(5), p 331–335
Acknowledgments
This project was financially supported by the National Natural Science Fund of China (51001049, 51271079), the Guangdong Natural Science Fund (2016A030313450, 2015A030313223) and the Fundamental Research Funds for the Central Universities (2013ZM033, 2015ZM065).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiao, D., Li, M., Ding, H. et al. Modification of Low-Alloy Steel Surface by High-Temperature Gas Nitriding Plus Tempering. J. of Materi Eng and Perform 27, 361–367 (2018). https://doi.org/10.1007/s11665-018-3141-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-018-3141-0