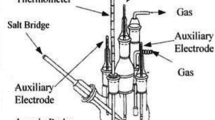
Abstract
The nature of surface oxide formed on carbon steel piping used in nuclear power plants affects flow-accelerated corrosion. In this investigation, carbon steel specimens were oxidized in an autoclave using demineralized water at various temperatures (150-300 °C) and at pH levels (neutral, 9.5). At low temperatures (< 240 °C), weight loss of specimens due to dissolution of iron in water occurred to a greater extent than weight gain due to oxide formation. With the increase in temperature, the extent of iron dissolution reduced and weight gain due to oxide formation increased. A similar trend was observed with the increase in pH as was observed with the increase in temperature. XRD and Raman spectroscopy confirmed the formation of magnetite. The oxide film formed by precipitation process was negligible at temperatures from 150 to 240 °C compared to that at higher temperatures (> 240 °C) as confirmed by scanning electron microscopy. Electrochemical impedance measurement followed by Mott–Schottky analysis indicated an increase in defect density with exposure duration at 150 °C at neutral pH but a low and stable defect density in alkaline environment. The defect density of the oxide formed at neutral pH at 150-300 °C was always higher than that formed in alkaline environment as reported in the literature.














Similar content being viewed by others
References
V. Kain, S. Roychowdhury, T. Mathew, and A. Bhandakkar, Flow Accelerated Corrosion and Its Control Measures for the Secondary Circuit Pipelines in Indian Nuclear Power Plants, J. Nucl. Mater., 2008, 383, p 86–91
V. Kain, S. Roychowdhury, P. Ahmedabadi, and D.K. Barua, Flow Accelerated Corrosion: Experience from Examination of Components from Nuclear Power Plants, Eng. Fail. Anal., 2011, 18, p 2028–2041
W.H. Ahmed, Evaluation of the Proximity Effect on Flow-Accelerated Corrosion, Ann. Nucl. Energy, 2010, 37, p 598–605
A.C.F. Guimaraes, A New Methodology for the Study of FAC Phenomenon Based on a Fuzzy Rule System, Ann. Nucl. Energy, 2003, 30, p 853–864
B. Poulson, Complexities in Predicting Erosion Corrosion, Wear, 1999, 233–235, p 497–504
R.B. Dooley and V.K. Chexal, Flow-Accelerated Corrosion of Pressure Vessels in Fossil Plants, Int. J. Prest, Ves. Pip., 2000, 77, p 85–90
J.H. Moon, H.H. Chung, K.W. Sung, U.C. Kim, and J.S. Rho, Dependency of Single-Phase FAC of Carbon and Low-Alloy Steels for NPP System Piping on pH, Orifice Distance and Material, Nucl. Eng. Technol., 2005, 37, p 375–384
V. Kain, Flow Accelerated Corrosion: Forms, Mech. Case Stud., Proc. Eng., 2014, 86, p 576–588
I. Betova, M. Bojinov, and T. Saario, Predictive Modelling of Flow Accelerated Corrosion-Unresolved Problems And Issues. VTT research report No VTT-R-08125-10, 2010.
F.H. Sweeton and C.F. Baes, The Solubility of Magnetite and Hydrolysis of Ferrous ion in Aqueous Solutions at Elevated Temperatures, J. Chem. Thermodyn., 1970, 2, p 479–500
P.R. Tremaine and J.C. LeBlanc, The Solubility of Magnetite and the Hydrolysis and Oxidation of Fe2 + in Water to 300 °C, J. Solut. Chem., 1980, 9, p 415–442
P.K. Sinha, M. Kiran Kumar, and V. Kain, Effect of Microstructure of Carbon Steel on Magnetite Formation in Simulated Hot Conditioning Environment of Nuclear Reactors, J. Nucl. Mater., 2015, 464, p 20–27
M. Kiran Kumar, K.B. Gaonkar, S. Ghosh, V. Kain, M. Bojinov, and T. Saario, Optimisation of the Hot Conditioning of Carbon Steel Surfaces of Primary Heat Transport System of Pressurized Heavy Water Reactors Using Electrochemical Impedance Spectroscopy, J. Nucl. Mater., 2010, 401, p 46–54
K. Mabuchi, Y. Horn, H. Takahashi, and M. Nagayama, Effect of Temperature and Dissolved Oxygen on the Corrosion Behavior of Carbon Steel in High-Temperature Water, Corrosion, 1991, 47, p 500–508
J. Wielant, V. Goossens, R. Hausbrand, and H. Terryn, Electronic properties of thermally formed thin iron oxide films, Electrochim. Acta, 2007, 52, p 7617–7625
ASTM G2/G2 M-06(2011) e1, Standard Test Method for Corrosion Testing of Products of Zirconium, Hafnium, and Their Alloys in Water at 680 °F (360 °C) or in Steam at 750 °F (400 °C), ASTM International, West Conshohocken, PA, 2011.
H.X. Guo, B.T. Lu, and J.L. Luo, Study on Passivation and Erosion-Enhanced Corrosion Resistance by Mott–Schottky Analysis, Electrochim. Acta, 2006, 52, p 1108–1116
Y.M. Zeng, J.L. Luo, and P.R. Norton, study of Semiconducting Properties of Hydrogen Containing Passive Films, Thin Solid Films, 2004, 460, p 116–124
H. Luo, C.F. Dong, X.Q. Cheng, K. Xiao, and X.G. Li, Electrochemical Behavior of 2205 Duplex Stainless Steel in NaCl Solution with Different Chromate Contents, J. Mater. Eng. Perform., 2012, 21, p 1283–1291
L. Jinlong and L.Hongyun, Effect of Temperature and Chloride Ion Concentration on Corrosion of Passive Films on Nano/Ultrafine Grained Stainless Steels, J. Mater. Eng. Perform., 2014, 23, p 4223–4229
M. Zhu and C.W. Du, A New Understanding on AC Corrosion of Pipeline Steel in Alkaline Environment, J. Mater. Eng. Perform., 2016, 26, p 221–228
W.P. Gomes and D. Vanmaekelbergh, Impedance Spectroscopy at Semiconductor Electrodes: Review and Recent Developments, Electrochim. Acta, 1996, 41, p 967–973
A.M.P. Simoes, M.G.S. Ferrira, and B. Rondot, Study of Passive Films Formed on AISI, 304 Stainless Steel by Impedance Measurements and Photoelectrochemistry, J. Electrochem. Soc., 1990, 137, p 82–87
ASTM A106/A106 M-15, Standard Specification for Seamless Carbon Steel Pipe for High-Temperature Service, ASTM International, West Conshohocken, PA, 2015,
J. Robertson, The Mechanism of High Temperature Aqueous Corrosion of Steel, Corros. Sci., 1989, 29, p 1275–1291
C. Frayne, Boiler Water Treatment Principles and Practice, Chemical Publishing Co. Inc, New York, 2002
R.O. Rihan and S. Nesic, Erosion–Corrosion of Mild Steel in Hot Caustic. Part I: NaOH Solution, Corros. Sci., 2006, 48, p 2633–2659
U. Schwertmann, R.M. Cornell, Iron Oxides in the Laboratory. Wiley-VCH, 2nd. ed. Weinheim, 2000, ISBN 3-527-29669-7.
K. Fujiwaraa, M. Domae, K. Yoneda, F. Inada, T. Ohira, and K. Hisamune, Correlation of Flow Accelerated Corrosion Rate with Iron Solubility, Nucl. Eng. Des., 2011, 241, p 4482–4486
D.D. Macdonald, et. al., The Thermodynamics of Metal-Water Systems at Elevated Temperatures. Part 2: The Iron Water System. AECL-4137.
M.A. Styrikovich et al., Solubility of Magnetite in Boiling Water at High Temperature, Teploenergetika, 1971, 18(7), p 82
MdA Islam and Z.N. Farhat, Characterization of the Corrosion Layer on Pipeline Steel in Sweet Environment, J. Mater. Eng. Perform., 2015, 24, p 3142–3158
O.N. Shebanova and P. Lazor, Raman Spectroscopic Study Of Magnetite (FeFe2O4): A New Assignment for the Vibrational Spectrum, J. Solid State Chem., 2003, 174, p 424–430
D.L.A. de Faria, S.V. de Silva, and M.T. Oliveira, Raman Microspectroscopy of Some Iron Oxides and Oxyhydroxides, J. Raman Spectrosc., 1997, 28, p 873–878
Y. El Mendili, A. Abdelouas, and J.-F. Bardeau, The Corrosion Behavior of Carbon Steel in Sulfide Aqueous Media at 30 °C, J. Mater. Eng. Perform., 2014, 23, p 1350–1357
M. Bojinov, K. Gaonkar, S. Ghosh, V. Kain, K. Kumar, and T. Saario, Characterisation of the Oxide Layer on Carbon Steel During Hot Conditioning of Primary Heat Transport Systems in Heavy-Water Reactors, Corros. Sci., 2009, 51, p 1146–1156
L. Zhang and D.D. Macdonald, On the Transport of Point Defects in Passive Fims, Electrochim. Acta, 1998, 43, p 679–691
D.D. Macdonald, Passivity—the Key to Our Metals-Based Civilization, Pure Appl. Chem., 1999, 71, p 951–978
S. Fujimoto and H. Tsuchiya, Semiconductor Properties and Protective Role of Passive Films of Iron Base Alloys, Corros. Sci., 2007, 49, p 195–202
M. Bojinov, G. Fabricius, T. Laitinen, K. Mäkelä, T. Saario, and G. Sundholm, Coupling Between Ionic Defect Structure and Electronic Conduction in Passive Films on Iron, Chromium and Iron–Chromium Alloys, Electrochim. Acta, 2000, 45, p 2029–2048
L. Hamadou, A. Kadri, and N. Benbrahim, Characterisation of Passive Films Formed on Low Carbon Steel in Borate Buffer Solution (pH 9.2) by Electrochemical Impedance Spectroscopy, Appl. Surf. Sci., 2005, 252, p 1510–1519
V. Subramanian, S. Chandran, H. Subramanian, P. Chandramohan, S. Bera, S. Rangarajan, and S.V. Narasimhan, Electrochemical Characterization of Oxide Formed on Chromium Containing Mild Steel Alloys in LiOH Medium, Mater. Chem. Phys., 2014, 145, p 499–509
X.-P. Guo, Y. Tomoe, H. Imaizumi, and K. Katoh, The Electrochemical Behavior and Impedance Characteristics of the Passive Film on Carbon Steel in Nitric Acid Solutions, J. Electroanal. Chem., 1998, 445, p 95–103
D. Macdonald, Some Personal Adventures in Passivity Da Review of the Point Defect Model for Film Growth, Russ. J. Electrochem., 2012, 48, p 235–258
F. La Mantia, H. Habazaki, M. Santamaria, and F. Di Quarto, A Critical Assessment of the Mott–Schottky Analysis for the Characterisation of Passive Film-Electrolyte Junctions, Russ. J. Electrochem., 2010, 46, p 1306–1322
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubey, V., Kain, V. Oxidation Behavior of Carbon Steel: Effect of Formation Temperature and pH of the Environment. J. of Materi Eng and Perform 26, 5312–5322 (2017). https://doi.org/10.1007/s11665-017-3027-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-017-3027-6