Abstract
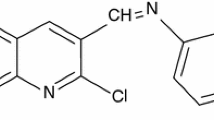
The corrosion inhibition of mild steel in 1 M HCl by 4-hydroxybenzaldehyde-1,3propandiamine (4-HBP) has been investigated using potentiodynamic polarization, electrochemical impedance spectroscopy and chronoamperometry measurements. The experimental results suggest that this compound is an excellent corrosion inhibitor for mild steel and the inhibition efficiency increases with the increase in inhibitor concentration. Polarization curves reveal that this organic compound is a mixed-type inhibitor. The effect of temperature on the corrosion behavior of mild steel in 1 M HCl with the addition of the Schiff base was studied in the temperature range from 25 to 65 °C. The experimentally obtained adsorption isotherms follow the Langmuir equation. Activation parameters and thermodynamic adsorption parameters of the corrosion process such as E a, ΔH, ΔS, K ads, and ΔG ads were calculated by the obtained corrosion currents at different temperatures and using the adsorption isotherm. The morphology of mild steel surface after its exposure to 1 M HCl solution in the absence and in the presence of 4-HBP was examined by AFM images.















Similar content being viewed by others
References
I. Ahamad, R. Prasad, and M.A. Quraishi, Thermodynamic, Electrochemical and Quantum Chemical Investigation of Some Schiff Bases as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid Solutions, Corros. Sci., 2010, 52, p 933–942
S. Issaadi, T. Douadi, A. Zouaoui, S. Chafaa, M.A. Khan, and G. Bouet, Novel Thiophene Symmetrical Schiff Base Compounds as Corrosion Inhibitor for Mild Steel in Acidic Media, Corros. Sci., 2011, 53, p 1484–1488
R. Solmaz, Investigation of the Inhibition Effect of 5-((E)-4-phenylbuta-1,3-dienylideneamino)-1,3,4-thiadiazole-2-thiol Schiff base on Mild Steel Corrosion in Hydrochloric Acid, Corros. Sci., 2010, 52, p 3321–3330
I. Ahamad, Experimental and Quantum Chemical Characterization of the Adsorption of Some Schiff Base Compounds of Phthaloylthiocarbohydrazide on the Mild Steel in Acid Solutions, Mater. Chem. Phys., 2010, 124, p 1155–1165
P. Lowmunkhong, D. Ungthararak, and P. Sutthivaiyakit, Tryptamine as a Corrosion Inhibitor of Mild Steel in Hydrochloric Acid Solution, Corros. Sci., 2010, 52, p 30–36
K.C. Emregu, E. Duzgun, and O. Atakol, The Application of Some Polydentate Schiff Base Compounds Containing Aminic Nitrogens as Corrosion Inhibitors for Mild Steel in Acidic Media, Corros. Sci., 2006, 48, p 3243–3260
S.M.A. Hosseini and A. Azimi, The Inhibition Effect of the New Schiff Base, Namely 2,20-[bis-N(4-choloro benzaldimin)]-1,10-dithio Against Mild Steel Corrosion, Mater. Corros., 2008, 59(1), p 41–45
R. Solmaz, E. Altunbas, and G. Kardas, Adsorption and Corrosion Inhibition Effect of 2-((5-Mercapto-1,3,4-thiadiazol-2-ylimino)methyl)phenol Schiff Base on Mild Steel, Mater. Chem. Phys., 2011, 125, p 796–801
A.M. Badiea and K.N. Mohana, Effect of Temperature and Fluid Velocity on Corrosion Mechanism of Low Carbon Steel in Presence of 2-Hydrazino-4,7-dimethylbenzothiazole in Industrial Water Medium, Corros. Sci., 2009, 51, p 2231–2241
K. Stanly Jacob and G. Parameswaran, Corrosion Inhibition of Mild Steel in Hydrochloric Acid Solution by Schiff Base Furointhiosemicarbazone, Corros. Sci., 2010, 52, p 224–228
R. Hasanov, Electrochemical and Quantum Chemical Studies of Some Schiff Bases on the Corrosion of Steel in H2SO4 Solution, Appl. Surf. Sci., 2007, 253, p 3913–3921
R.A. Prabhu, T.V. Venkatesha, A.V. Shanbhag, G.M. Kulkarni, and R.G. Kalkhambkar, Inhibition Effects of Some Schiff Bases on the Corrosion of Mild Steel in Hydrochloric Acid Solution, Corros. Sci., 2008, 50, p 3356–3362
S.A. Fairhurst, D.L. Hughes, U. Kleinkes, G.J. Leigh, J.R. Sanders, J. Weisner, Non-planar Co-ordination of the Schiff-Based Anion N,N′-2,2Dimethyltrimethylenebis [salicylideneiminate(2–)] to Vanadium, J. Chem. Soc. Dalton Trans., 1995, p 321–326
P. Geerlings, F.D. Proft, and W. Langenaeker, Conceptual Density Functional Theory, Chem. Rev., 2003, 103, p 1793–1873
C. Lee, W. Yang, and R.G. Parr, Development of the Colle-Salvetticonelation Energy Formula into a Functional of the Electron Density, Phys. Rev. B, 1988, 37, p 785–789
G.A. Petersson and M.A. Al-Laham, A Complete Basis Set Model Chemistry. II, Open-shell Systems and the Total Energy of the First-Row Atoms, J. Chem. Phys., 1991, 94, p 6081–6086
G.A. Petersson, A. Bennett, T.G. Tensfeldt, M.A. Al-Laham, W.A. Shirley, and J. Mantzaris, A Complete Basis Set Model Chemistry. I. The Total Energies of Closed-Shell Atoms and Hydrides of the First-Row Elements, J. Chem. Phys., 1988, 89, p 2193–2219
J.R. MacDonald, Note on the Parameterization of the Constant-Phase Admittance Element, Solid State Ion., 1984, 13, p 147–149
I. Danaee, M. Jafarian, F. Forouzandeh, F. Gobal, and M.G. Mahjani, Kinetic Interpretation of a Negative Time Constant Impedance of Glucose Electrooxidation, J. Phys. Chem. B, 2008, 112, p 15933–15940
N.A. Negm, Y.M. Elkholy, M.K. Zahran, and S.M. Tawfik, Corrosion Inhibition Efficiency and Surface Activity of Benzothiazol-3-ium Cationic Schiff Base Derivatives in Hydrochloric Acid, Corros. Sci., 2010, 52, p 3523–3536
N.A. Negm, F.M. Ghuiba, and S.M. Tawfik, Novelis Oxazolium Cationic Schiff Base Compounds as Corrosion Inhibitors for Carbon Steel in Hydrochloric Acid, Corros. Sci., 2011, 53, p 3566–3575
M.A. Hegazy, A Novel Schiff Base-based Cationic Gemini Surfactants: Synthesis and Effect on Corrosion Inhibition of Carbon Steel in Hydrochloric Acid Solution, Corros. Sci., 2009, 51, p 2610–2618
K. Mallaiya, R. Subramaniam, and S.S. Srikandan, Electrochemical Characterization of the Protective Film Formed by the Unsymmetrical Schiff’s Base on the Mild Steel Surface in Acid Media, Electrochim. Acta, 2011, 56, p 3857–3863
M.A. Migahed and I.F. Nassar, Corrosion Inhibition of Tubing Steel During Acidization of Oil and Gas Wells, Electrochim. Acta, 2008, 53, p 2877–2882
H. Keles, Electrochemical and Thermodynamic Studies to Evaluate Inhibition Effect of 2-[(4-Phenoxy-phenylimino) methyl]-phenol in 1 M HCl on Mild Steel, Mater. Chem. Phys., 2011, 130, p 1317–1324
K.C. Emregül and O. Atakol, Corrosion Inhibition of Mild Steel with Schiff Base Compounds in 1 M HCl, Mater. Chem. Phys., 2003, 82, p 188–193
I. Danaee, Kinetics and Mechanism of Palladium Electrodeposition on Graphite Electrode by Impedance and Noise Measurements, J. Electroanal. Chem., 2011, 662, p 415–420
I. Danaee and S. Noori, Kinetics of the Hydrogen Evolution Reaction on NiMn Graphite Modified Electrode, Int. J. Hydrogen Energy, 2011, 36, p 12102–12111
F. Bentiss, M. Traisnel, H. Vezin, H.F. Hildebrand, and M. Lagrenee, 2,5-Bis(4-dimethylaminophenyl)-1,3,4-oxadiazole and 2,5-bis(4-dimethylaminophenyl)-1,3,4-thiadiazole as Corrosion Inhibitors for Mild Steel in Acidic Media, Corros. Sci., 2004, 46, p 2781–2792
R.A. Prabhu, T.V. Venkatesha, A.V. Shanbhag, B.M. Praveen, G.M. Kulkarni, and R.G. Kalkhambkar, Quinol-2-thione Compounds as Corrosion Inhibitors for Mild Steel in Acid Solution, Mater. Chem. Phys., 2008, 108, p 283–289
S. Zhang, Z. Tao, S. Liao, and F. Wu, Substitutional Adsorption Isotherms and Corrosion Inhibitive Properties of Some Oxadiazol-Triazole Derivative in Acidic Solution, Corros. Sci., 2010, 52, p 3126–3132
G.E. Badr, The Role of Some Thiosemicarbazide Derivatives as Corrosion Inhibitors for C-Steel in Acidic Media, Corros. Sci., 2009, 51, p 2529–2536
M.A. Hegazy, H.M. Ahmed, and A.S. El-Tabei, Investigation of the Inhibitive Effect of p-Substituted4-(N,N,N-dimethyldodecylammonium bromide)benzylidene-benzene-2-yl-amineon Corrosion of Carbon Steel Pipelines in Acidic Medium, Corros. Sci., 2011, 53, p 671–678
J. Aljourani, K. Raeissi, and M.A. Golozar, Benzimidazole and Its Derivatives as Corrosion Inhibitors for Mild Steel in 1 M HCl Solution, Corros. Sci., 2009, 51, p 1836–1843
I.B. Obot and N.O. Obi-Egbedi, Anti-corrosive Properties of Xanthone on Mild Steel Corrosion in Sulphuric Acid: Experimental and Theoretical Investigations, Curr. Appl. Phys., 2011, 11, p 382–392
L. Herrag, A. Chetouani, S. Elkadiri, B. Hammouti, and A. Aouniti, Pyrazole Derivatives as Corrosion Inhibitors for Steel in Hydrochloric Acid, Portugal. Ectrochim. Acta, 2008, 26, p 211–220
E. Bayol, T. Gurtenb, A.A. Gurtena, and M. Erbil, Interactions of Some Schiff Base Compounds with Mild Steel Surface in Hydrochloric Acid Solution, Mater. Chem. Phys., 2008, 112, p 624–630
H. Keles, M. Keles, I. Dehri, and O. Serindag, The Inhibitive Effect of 6-Amino-m-cresol and its Schiff Base on the Corrosion of Mild Steel in 0.5 M HCl Medium, Mater. Chem. Phys., 2008, 112, p 173–179
H. Keles, M. Keles, I. Dehri, and O. Serindag, Adsorption and Inhibitive Properties of Aminobiphenyl and its Schiff Base on Mild Steel Corrosion in 0.5 M HCl Medium, Colloids Surf. A, 2008, 320, p 138–145
S. Ghareba and S. Omanovic, Interaction of 12-Aminododecanoic Acid with a Carbon Steel Surface: Towards the Development of ‘green’ Corrosion Inhibitors, Corros. Sci., 2010, 52, p 2104–2113
X. Li and G. Mu, Tween-40 as Corrosion Inhibitor for Cold Rolled Steel in Sulphuric Acid: Weight Loss Study, Electrochemical Characterization, and AFM, Appl. Surf. Sci., 2005, 252, p 1254–1265
F. Zhang, J. Pan, and P.M. Claesson, Electrochemical and AFM Studies of Mussel Adhesive Protein (Mefp-1) as Corrosion Inhibitor for Carbon Steel, Electrochim. Acta, 2011, 56, p 1636–1645
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghasemi, O., Danaee, I., Rashed, G.R. et al. The Inhibition Effect of Synthesized 4-Hydroxybenzaldehyde-1,3propandiamine on the Corrosion of Mild Steel in 1 M HCl. J. of Materi Eng and Perform 22, 1054–1063 (2013). https://doi.org/10.1007/s11665-012-0348-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-012-0348-3