Abstract
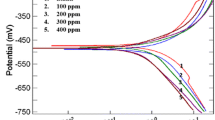
The inhibition of mild steel corrosion in aerated 0.5 N H2SO4 solution was investigated using potentiodynamic polarization studies (Tafel), linear polarization studies, electrochemical impedance spectroscopy studies, adsorption studies, and surface morphological studies. The effect of inhibitor concentration on corrosion rate, the effect of temperature, degree of surface coverage, adsorption kinetics, and surface morphology are investigated. The inhibition efficiency increased markedly with increase in the additive concentration and decreased slightly with increasing temperature. The presence of DMSTT decrease the double-layer capacitance and increase the charge transfer resistance. The value of activation energy (E a) of metal corrosion, adsorption equilibrium constant (K ads), and free energy of adsorption (ΔG ads) were calculated from the temperature dependence of corrosion current. The adsorption of inhibitor molecule on mild steel surface follow Langmuir isotherm. DMSTT offers excellent inhibition properties and acts as a mixed-type inhibitor.









Similar content being viewed by others
References
S. John, B. Joseph, K.K. Aravindakshan, and A. Joseph, Inhibition of Mild Steel Corrosion in 1 M Hydrochloric Acid by 4-(N,N-Dimethylaminobenzylidine)-3-mercapto-6-methyl-1,2,4-triazin(4H)-5-one, Mater. Chem. Phys., 2010, 122, p 374–379
S. John, B. Joseph, K.V. Balakrishnan, K.K. Aravindakshan, and A. Joseph, Electrochemical and Quantum Chemical Study of 4-[(E)-[(2,4-Dihydroxy phenyl) methylidene] amino]-6-methyl-3-sulphanylidine-2,3,4,5-tetrahydro-1,2,4-triazin-5-one, Mater. Chem. Phys., 2010, 123, p 218–224
A. Fiala, A. Chibani, A. Darchen, A. Boulkamh, and K. Djebbar, Investigations of the Inhibition of Copper Corrosion in Nitric Acid Solutions by Ketene Dithioacetal Derivatives, Appl. Surf. Sci., 2007, 253, p 9347–9356
F. Zucchi, G. Trabanelli, and C. Monticelli, The Inhibition of Copper Corrosion in 0.1 M NaCl Under Heat Exchange Conditions, Corros. Sci., 1996, 38, p 147–154
J.P. Chopart, J. Douglade, P. Fricoteaux, and A. Olivier, Electrodeposition and Electrodissolution of Copper with a Magnetic Field: Dynamic and Stationary Investigations, Electrochim. Acta, 1991, 36, p 459–463
M.D. Pritzker and T.Z. Fahidy, Morphological Stability of a Planar Metal Electrode During Potentiostatic Electrodeposition and Electrodissolution, Electrochim. Acta, 1992, 37, p 103–112
S. Magaino, Corrosion Rate of Copper Rotating-Disk-Electrode in Simulated Acid Rain, Electrochim. Acta, 1997, 42, p 377–382
S. Krzewska, Impedance Investigation of the Mechanism of Copper Electrodeposition from Acidic Perchlorate Electrolyte, Electrochim. Acta, 1997, 42, p 3531–3540
M.U. Macdonald, S. Silvia Real, and D.D. Macdonald, Application of Kramers-Kronig Transforms in the Analysis of Electrochemical Impedance Data, J. Electrochem. Soc., 1986, 133, p 2018–2024
J. Bastidas, J. De Damborenea, and A.J. Va Zquez, Butyl Substituents in n-Butylamine and Their Influence on Mild Steel Corrosion Inhibition in Hydrochloric Acid, J. Appl. Electrochem., 1997, 27, p 345–349
E.E. Ebenso, T. Arslan, F. Kandemirli, N. Caner, and I. Love, Quantum Chemical Studies of Some Rhodanine Azosulpha Drugs as Corrosion Inhibitors for Mild Steel in Acidic Medium, Int. J. Quantum Chem., 2010, 110, p 1003–1018
G. Gece, The Use of Quantum Chemical Methods in Corrosion Inhibitor Studies, Corros. Sci., 2008, 50, p 2981–2992
R. Solmaz, G. Kardas, M. Culha, B. Yazici, and M. Erbil, Investigation of Adsorption and Inhibitive Effect of 2-Mercaptothiazoline on Corrosion of Mild Steel in Hydrochloric Acid Media, Electrochim. Acta, 2008, 53, p 5941–5952
R. Fuchs-Godec, The Adsorption CMC Determination and Corrosion Inhibition of Some N-Alkyl Quaternary Ammonium Salts on Carbon Steel Surface in 2 M H2SO4, Colloids. Surf. A, 2006, 280, p 130–139
A. Chetouani, B. Hammouti, T. Benhadda, and M. Daoudi, Inhibitive Action of Bipyrazolic Type Organic Compounds Towards Corrosion of Pure Iron in Acidic Media, Appl. Surf. Sci., 2005, 249, p 375–380
N. Labjar, M. Lebrini, F. Bentiss, N.E. Chihib, S. Hajjaji, and C. Jama, Corrosion Inhibition of Carbon Steel and Antibacterial Properties of Aminotris-(methylenephosphonic) Acid, Mater. Chem. Phys., 2010, 119, p 330–336
M.A. Amin, S.S. Abed El-Rehim, E.E.F. El-Sherbini, and R.S. Bayyomi, The Inhibition of Low Carbon Steel Corrosion in Hydrochloric Acid Solutions by Succinic Acid: Part I. Weight Loss, Polarization, EIS, PZC, EDX and SEM Studies, Electrochim. Acta, 2007, 52, p 3588–3600
M.A. Veloz and I. Gonzalez, Electrochemical Study of Carbon Steel Corrosion in Buffered Acetic Acid Solutions with Chlorides and H2S, Electrochim. Acta, 2002, 48, p 135–144
E.M. Sherif and S.M. Park, Effects of 1,4-Naphthoquinone on Aluminum Corrosion in 0.50 M Sodium Chloride Solutions, Electrochim. Acta, 2006, 51, p 1313–1321
F. Mansfeld and M.W. Kendig, Determination of the Polarization Resistance from Impedance Measurements, Werkst. Korros., 1983, 34, p 397–401
R. Macdonald and D.R. Franceshetti, Impedance Spectroscopy, Wiley, New York, 1987
M. Outirite, M. LagreneeLebrini, M. Traisnel, C. Jama, H. Vezin, and F. Bentiss, AC Impedance, X-Ray Photoelectron Spectroscopy and Density Functional Theory Studies of 3,5-bis(n-pyridyl)-1,2,4-oxadiazoles as Efficient Corrosion Inhibitors for Carbon Steel Surface in Hydrochloric Acid Solution, Electrochim. Acta, 2010, 55, p 1670–1681
D.A. Lopez, S.N. Simison, and S.R. de Sanchez, The Influence of Steel Microstructure on CO2 Corrosion. EIS Studies on the Inhibition Efficiency of Benzimidazole, Electrochim. Acta, 2003, 48, p 845–854
K.F. Khaled and M.M. Al-Qahtani, The Inhibitive Effect of Some Tetrazole Derivatives Towards Al Corrosion in Acid Solution: Chemical, Electrochemical and Theoretical Studies, Mater. Chem. Phys., 2009, 113, p 150–158
M.R. Saleh and A.M. Shams El Din, Efficiency of Organic Acids and Their Anions in Retarding the Dissolution of Aluminium, Corros. Sci., 1981, 12, p 688–697
J. Fang and J. Li, Quantum Chemistry Study on the Relationship Between Molecular Structure and Corrosion Inhibition Efficiency of Amides, J. Mol. Struct. (Theochem), 2002, 593, p 179–185
M.K. Awad, R.M. Issa, and F.M. Atlam, Theoretical Investigation of the Inhibition of Corrosion by Some Triazole Schiff Bases, Mater. Corros., 2009, 60, p 813–819
W. Li, X. Zhao, F. Liu, J. Deng, and B. Hou, Investigation on the Corrosion Inhibitive Effect of 2H-Pyrazole-Triazole Derivatives in Acidic Solution, Mater. Corros., 2009, 60, p 287–293
R. Hasanov, M. Sadikoglu, and S. Bilgic, Electrochemical and Quantum Chemical Studies of Some Schiff Bases on the Corrosion of Steel in H2SO4 Solution, Appl. Surf. Sci., 2007, 253, p 3913–3921
M.A. Amin, K.F. Khaledand, and S.A. Fadl-Allah, Testing Validity of the Tafel Extrapolation Method for Monitoring Corrosion of Cold Rolled Steel in HCl Solutions—Experimental and Theoretical Studies, Corros. Sci., 2010, 52, p 140–151
H. wang, X. Wang, H. Wang, L. Wang, and A. Liu, DFT Study of New Bipyrazole Derivatives and Their Potential Activity as Corrosion Inhibitors, J. Mol. Model., 2007, 1, p 147–153
K.F. Khaled and M.A. Amin, Corrosion Monitoring of Mild Steel in Sulphuric Acid Solutions in Presence of Some Thiazole Derivatives—Molecular Dynamics, Chemical and Electrochemical Studies, Corros. Sci., 2009, 51, p 1964–1975
S. Xia, M. Qiu, L. Yu, F. Liu, and H. Zhao, Molecular Dynamics and Density Functional Theory Study on Relationship Between Structure of Imidazoline Derivatives and Inhibition Performance, Corros. Sci., 2008, 50, p 2021–2029
S.V. Ramesh and A.V. Adhikari, N-[4-(Diethylamino)benzylidine]-3-{[8-(trifluoromethyl) quinolin-4-yl]thio}propano hydrazide) as an Effective Inhibitor of Mild Steel Corrosion in Acid Media, Mater. Chem. Phys., 2009, 115, p 618–627
K. Babic-Samardzija, C. Lupu, N. Hackerman, and A.R. Barron, Inhibitive Properties, Adsorption and Surface Study of Butyn-1-ol and pentyn-1-ol Alcohols as Corrosion Inhibitors for Iron in HCl, J. Mater. Chem., 2005, 15, p 1908–1916
P. Zhao, Q. Liang, and Y. Li, Electrochemical, SEM/EDS and Quantum Chemical Study of Phthalocyanines as Corrosion Inhibitors for Mild Steel in 1 mol/l HCl, Appl. Surf. Sci., 2005, 252, p 1596–1607
H.L. Wang, H.B. Fan, and J.S. Zheng, Corrosion Inhibition of Mild Steel in Hydrochloric Acid Solution by a Mercapto-Triazole Compound, Mater. Chem. Phys., 2002, 77, p 655–661
A.K. Singh and M.A. Quraishi, Inhibiting Effects of 5-Substituted Isatin-Based Mannich Bases on the Corrosion of Mild Steel in Hydrochloric Acid Solution, J. Appl. Electrochem., 2010, 40, p 1293–1306
Acknowledgment
Sam John is grateful to CSIR New Delhi for providing senior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
John, S., Joseph, A. Corrosion Protection Properties of 4-[(E)-[(2,4-Dihydroxy phenyl)methylidene] amino]-6-methyl-3-sulfanylidene-2,3,4,5-tetrahydro-1,2,4-triazin-5-one [DMSTT] Toward Mild Steel in Sulfuric Acid. J. of Materi Eng and Perform 22, 483–491 (2013). https://doi.org/10.1007/s11665-012-0292-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-012-0292-2