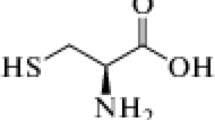
Abstract
The inhibitory effects of tryptophan on the corrosion of AA 2024 in 1 M HCl, 20% (wt.%) CaCl2, and 3.5% (wt.%) NaCl solutions were investigated via polarization techniques, electrochemical impedance spectroscopy, and weight loss methods. The scanning electron microscope technique was employed to observe corrosion morphology. The results suggest that AA 2024 was corroded in these three corrosive media to some extent and that tryptophan can significantly inhibit the corrosion of aluminum alloys. The inhibition efficiency (η) increased with increasing concentrations of tryptophan, and the best inhibition efficiency exhibited was about 87% in 1 M HCl solution with 0.008 M tryptophan. Tryptophan acted as a cathodic corrosion inhibitor and affected the hydrogen evolution reaction, which was the main electrode reaction in the 1 M HCl solution. In solutions with 20% CaCl2 and 3.5% NaCl, tryptophan was adsorbed onto anodic areas, thus increasing the activation energy of the interface reaction as an anodic corrosion inhibitor. The Dmol3 program of Material Studio 4.0 was used to obtain the optimized geometry of the tryptophan inhibitor and some quantum-chemical parameters. Front orbital distributions and Fukui indices indicate that the molecular active reaction zones were located in the indole ring of tryptophan.





Similar content being viewed by others
References
S. Wernick, R. Pinner, and P.G. Sheasby, The Surface Treatment of Aluminum and its Alloys, Vol 1, ASN International Finishing Publications Ltd., Teddington, UK, 1987
C.-h. Yu, The Analysis on Chemical Cleaning Agent in Aluminum Fin Cool-Exchanger, Chem. Clean., 1999, 15(2), p 12–16
D.-x. Yu, Preparation of a Normal Temperature High Efficiency Cleaner for Aluminum Slices, Fine Chem., 2003, 20(2), p 126–128
Q.-z. Joao, Study on the Inhibitor of SH-904 and JA-1 on Aluminum in Hydrochloric Acid Solution, J. Sci. Teachers’ Coll. Univ., 2004, 2(44), p 23–25
N.-l. Wang and X.-b. Liu, Prevention Analysis of Salt-Spray Corrosion Coating of Aluminium Heat Exchanger, Mech. Manag. Develop., 2007, 6(3), p 33–35
J.-y. Zuo, Stress Corrosion Crack, Xi’an Jiao Tong University Press, Xi’an, 1985, p 222–225
Y.-l. Wang and J.-s. Wang, Corrosion Analysis of Heat Exchanger in Electric Desalter of Delay Cooking Unit, Mater. Mech. Eng., 2008, 1(32), p 81–83
Z. Mu and S.-w. Jing, Corrosion and Prevent of Heat Exchanger, Large Scale Nitrogenous Fertilizer Industry, 2008, 5(3), p 38–40
A.A. El-Shafei, M.N.H. Moussa, and A.A. El-far, Inhibitory Effect of Amino Acids on Al Pitting Corrosion in 0.1 M NaCl, J. Appl. Electrochem., 1997, 27(9), p 1075–1078
X.-k. Yang, Preparation of the Pickling Inhibitor of Complex Amino Acid, New Technol. New Process, 2002, 10, p 39–40
H. Ashassi-Sorkhabi, M.R. Majidi, and K. Seyyedi, Investigation of Inhibition Effect of Some Amino Acids Against Steel Corrosion in HCl Solution, Appl. Surf. Sci., 2004, 225(1/4), p 176–185
E.E. Oguzie, Y. Li, and F.H. Wang, Corrosion Inhibition and Adsorption Behavior of Methionine on Mild Steel in Sulfuric Acid and Synergistic Effect of Iodide Ion [J], J. Colloid Interface Sci., 2007, 310(1), p 90–98
M.A. Kiani, M.F. Mousavi, S. Ghasemi, M. Shamsipur, and S.H. Kazemi, Inhibitory Effect of Some Amino Acids on Corrosion of Pb-Ca-Sn Alloy in Sulfuric Acid Solution, Corros. Sci., 2008, 50(4), p 1035–1045
Metal Materials—Uniform Corrosion—Methods of Laboratory Immersion Testing. GB10124-88, Chinese National Standard, 1990, p 119–126
S.-z. Song, Electrochemical Methods for Corrosion Investigation[M], Chemical Industry Press, Beijing, 1988, p 164–165
I. Epelboin and M. Keddam, Faradic Impedance: Diffusion Impedance and Reaction Impedance, Electrochem. Soc., 1970, 117, p 1052–1061
M. Keddam, O.R. Mattos, and H. Takenouti, Reaction Model for Iron Diffusion Studied by Electrode Impedance, Electrochem. Soc., 1959, 55, p 1562–1586
R.G. Parr and W. Yang, Density Functional Approach to the Frontier-electron Theory of Chemical Reactivity, J. Am. Chem. Soc., 1984, 106(14), p 4049–4050
W. Yang and W.J. Mortier, The Use of Global and Local Molecular Parameters for the Analysis of the Gas-phase Basicity of Amines, J. Am. Chem. Soc., 1986, 108(19), p 5708–5713
B. Gómez, N.V. Likhanova, M.A. Domínguez-Aguilar, R. Martínez-Palou, A. Vela, and J.L. Gazguez, Quantum Chemical Study of the Inhibitive Properties of 2-Pyridyl-Azoles, J. Phys. Chem. B, 2006, 110(18), p 8928–8937
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Xiang, B., Zuo, Xl. et al. Inhibition of Tryptophan on AA 2024 in Chloride-Containing Solutions. J. of Materi Eng and Perform 20, 265–270 (2011). https://doi.org/10.1007/s11665-010-9680-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-010-9680-7