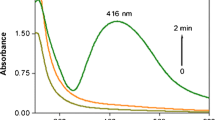
Silver nanoparticles of narrow size distributions were synthesized by reduction of highly concentrated silver nitrate (AgNO3) solution with sodium hypophosphite (NaH2PO2 · H2O) in the presence of polyvinyl pyrrolidone (PVP). An orthogonal experimental design (OED) with L9 orthogonal array was employed as a chemometric method to optimize the experimental conditions for the synthesis of silver nanoparticles. Particle size of silver nanoparticles was considered as the defining characteristics. The concentration of reducing agent, weight ratio of AgNO3 to protecting agent, and temperature were optimized using a three-level OED and nine experiments. The particle size was characterized to optimize the synthesis conditions. The concentration of reducing agent emerged as the most important parameter influencing the particle size. The temperature also influenced the particle size. Based on 1 M AgNO3 solution and 0.1 M NaH2PO2 · H2O, 1.7 g silver nanoparticles of 10-50 nm were obtained from 3.4 g silver nitrate with weight ratio of PVP/AgNO3 equal to unity at 40 °C.





Similar content being viewed by others
References
Y. Sun, Y. Xia, Large-scale synthesis of uniform silver nanowires through a soft, self-seeding, polyol process, Adv. Mater., 14, 2002, p 833-837.
W.J. Yan, R. Wang, Z.Q. Xu, J.K. Xu, L. Lin, Z.Q. Shen, and Y.F. Zhou, A novel, practical and green synthesis of Ag nanoparticles catalyst and its application in three-component coupling of aldehyde, alkyne, and amine, J. Mol. Catal. A: Chem., 255, 2006, p 81–85.
[3] D. Poondi, T. Dobbins, J. Singh, Novel laser-liquid-solid interaction technique for synthesis of silver, nickel and immiscible silver-nickel alloys from liquid precursors, J. Mater. Sci., 35, 2000, p 6237-6243.
W.T. Cheng, Y.W. Chih, and W.T. Yeh, In situ fabrication of photocurable conductive adhesives with silver nano-particles in the absence of capping agent, In. J. Adhes. Adhes., 27, 2007, p 236-243.
K. Aslan, I. Gryczynski, J. Malicka, E. Matveeva, J.R. Lakowicz, C.D. Geddes, Metal-enhanced fluorescence: An emerging tool in biotechnology, Curr. Opin. Biotechnol., 16, 2005, p 55-62.
K.S. Chou, C.Y. Ren, Synthesis of nanosized silver particles by chemical reduction method, Mater. Chem. Phys., 64, 2000, p 241-246.
P.K. Khanna, V.V.V.S. Subbarao, Nanosized silver powder via reduction of silver nitrate by sodium formaldehydesulfoxylate in acidic pH medium, Mater. Lett., 57, 2003, p 2242-2245.
H.S. Wang, X.L. Qiao, J.G. Chen, and S.Y. Ding, Preparation of silver nanoparticles by chemical reduction method, Colloids Surf. A: Physicochem Eng. Aspects, 256, 2005, p 111-115.
H.Y. Jia, J.B. Zeng, W. Song, J. An, B. Zhao (2006) Preparation of silver nanoparticles by photo-reduction for surface-enhanced Raman scattering, Thin Solid Films, 496:281-287.
L.C. Courrol, F.R.O. Silva, and L. Gomes, A simple method to synthesize silver nanoparticles by photo-reduction, Colloids Surf A: Physicochem. Eng. Aspects, 305, 2007, p 54-57.
Y.A. Kotov, O.M. Samatov, Production of nanometer-sized AlN powders by the exploding wire method, Nanostruct Mater., 12, 1999, p 119–122.
K. Okitsu, Y. Mizukoshi, H. Bandow, Y. Maeda, T. Yamamoto, and Y. Nagata, Formation of noble metal particles by ultrasonic irradiation, Ultrason. Sonochem., 3, 1996, p 249-251.
S.H. Lee, S.M. Oh, and D.W. Park, Preparation of silver nanopowder by thermal plasma, Mater. Sci. Eng. C, 27, 2007, p 1286-1290.
I. Sondi, D.V. Goia, and E. Matijevic, Preparation of highly concentrated stable dispersions of uniform silver nanoparticles, J. Colloid Interface Sci. 260, 2003, p 75-81.
K.D. Kim, D.N. Han, and H.T. Kim, Optimization of experimental conditions based on the Taguchi robust design for the formation of nano-sized silver particles by chemical reduction method, Chem. Engin. J., 104, 2004, p 55-61.
A. Dey, Orthogonal Fractional Factorial Designs. Wiley, USA: New York, 1985, p 1-133.
D.M. Gu, M.Y. Sun, Reaction mechanism of sodium hypophosphite in preparation of nanosize metal, J. Harbin Institute of Technology, 35, 2003, p 1009-1011 (in Chinese).
H.S. Wang, X.L Qiao, J.G. Chen, X.J. Wang, S.Y. Ding, Mechanisms of PVP in the preparation of silver nanoparticles, Mater. Chem. Phys., 94, 2005, p 449-453.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Z., Wang, Y. & Yu, Q. Significant Parameters in the Optimization of Synthesis of Silver Nanoparticles by Chemical Reduction Method. J. of Materi Eng and Perform 19, 252–256 (2010). https://doi.org/10.1007/s11665-009-9486-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-009-9486-7