Abstract
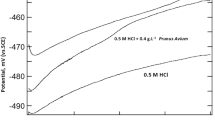
The effects of radish leaves and black cumin as plant extracts on the corrosion behavior of low-carbon steel in industrial water in the temperature range of 30 to 80 °C and velocity range of 1.44 to 2.02 m s−1 using potentiodynamic polarization, electrochemical impedance spectroscopy, and mass loss measurements have been investigated. The inhibition efficiency increased with increasing concentration of the plant extracts up to a critical value but it slightly decreased with increasing temperature. Inhibition efficiency values obtained from mass loss and potentiodynamic data were in reasonable agreement. Potentiodynamic polarization clearly indicated that radish leaves and black cumin extracts acted as anodic inhibitors. The adsorption behavior was found to obey the Flory-Huggins isotherm model. The associated activation parameters and thermodynamic data of adsorption were evaluated and discussed. The results show that radish leaves and black cumin could serve as effective inhibitors for low-carbon steel in industrial water media, with black cumin providing better protection than radish leaves.










Similar content being viewed by others
References
A. M. Abdel-Gaber, B.A. Abd-El-Nabey, I.M. Sidahmed, A.M. El-Zayady, M. Saadawy, “Inhibitive Action of Some Plant Extracts on the Corrosion of Steel in Acidic Media”, Corros. Sci. 48 (2006) 2765–2779
R. M. Saleh, A.A. Ismail, A.A. El-Hosary, “Corrosion Inhibition by Naturally Occurring Substances. VII. The Effect of Aqueous Extracts of Some Leaves and Fruit Peels on the Corrosion of Steel, Aluminum, Zinc and Copper in Acids”, Br. Corros. J. 17 (1982) 131–135.
K. Srivatsava, P. Srivatsava, “Studies on Plant Materials as Corrosion Inhibitors”, Br. Corros. J. 16 (1981) 221–223
A. Y. El-Etre, “Natural Honey as Corrosion Inhibitor for Metals and Alloys. I. Copper in Neutral Aqueous Solution”, Corros. Sci. 40 (1998) 1845–1850
A. Y. El-Etre, M. Abdallah, “Natural Honey as Corrosion Inhibitor for Metals and Alloys. II. C Steel in High Saline Water”, Corros. Sci. 42 (2000) 731–738
E. Khamis, N. Al-Andis, “Herbs as New Type of Green Inhibitors for Acidic Corrosion of Steel”, Materwiss. Werkstofftech. 33 (2002) 550–554
A. Chetouani, B. Hammouti, “Corrosion Inhibition of Iron in Hydrochloric Acid Solutions by Naturally Henna”, Bull. Electrochem. 19 (2003) 23–25
B. Hammouti, S. Kertit, M. Melhaoui, “Bgugaine: A Natural Pyrolidine Alkaloid Product as Corrosion Inhibitor of Iron in HCl Medium”, Bull. Electrochem. 11 (1995) 553–555
B. Hammouti, S. Kertit, M. Mellhaoui, “Electrochemical Behaviour of Gugaine as Corrosion Inhibitor of Iron in HCl Medium”, Bull. Electrochem. 13 (1997) 97–98
M. K. Gomma, M.H. Wahdan, “Schiff Bases as Corrosion Inhibitors for Aluminum in Hydrochloric Acid Solution”, Mater. Chem. Phys. 39 (1995) 209–213
M. G. Fontana, N. D. Green, Corrosion Engineering, 2nd ed., McGraw-Hill, New York, 1978
M.A. Ameer, E. Khamis, G. Al-Senani, “Effect of Temperature on Stability of Adsorbed Inhibitors on Steel in Phosphoric Acid Solution”, J. Appl. Electrochem. 32 (2002) 149–156
B. A. Abd-ENabey, E. Khamis, M.Sh. Ramadan. A. El-Gindy, Application of the Kinetic-Thermodynamic Model for Inhibition of Acid Corrosion of Steel by Inhibitors Containing Sulfur and Nitrogen, Corrosion 52 (1996) 671–679
N. H. Helal, M.M. El-Rabiee, Gh.M. Abd El-Hafez, W.A. Badawy, “Environmentally Safe Corrosion Inhibition of Pb in Aqueous Solutions”, J. Alloys Compd. 456 (2008) 372–379
J. Cruz, R. Martínez, J. Genesca, E. García-Ochoa, “Experimental and Theoretical Study of 1-(2-Ethylamino)-2-methylimidazoline as an Inhibitor of Carbon Steel Corrosion in Acid Media”, J. Electroanal. Chem. 566 (2004) 111–121
Hosseini, M., Mertens, S.F.L. and Ghorbani, M., M.R. Arshadi, “Asymmetrical Schiff Bases as Inhibitors of Mild Steel Corrosion in Sulphuric Acid Media” Mater. Chem. Phys. 78 (2003) 800–808
O. Benali, L. Larabi, M. Traisnel, L. Gengembre, Y. Harek, “Electrochemical, Theoretical and XPS Studies of 2-Mercapto-1-methylimidazole Adsorption on Carbon Steel in 1 M HClO4” Y. Harek, Appl. Surf. Sci., 253 (2007) 6130–6139
F. Bentiss, M. Lagrenée, M.Traisnel, J. C.Hornez, “Corrosion Inhibition of Mild Steel in 1 M Hydrochloric Acid by 2,5-Bis(2-Aminophenyl)-1,3,4-Oxadiazole” Corrosion 55 (1999) 968–976
T. F. Tadros, Applied Surfactants, 3rd ed., Wiley-VCH, Weinheim, 2005, p.87
E. E. Oguzie, C. Unaegbu, C.N. Ogukwe, B.N. Okolue, A.I. Onuchukwu, “Inhibition of Mild Steel Corrosion in Sulphuric Acid Using Indigo Dye and Synergistic Halide Additives”, Mater. Chem. Phys. 84 (2004) 363–368
S.S. Abd El Rehim, M.A.M. Ibrahim, K.F. Khalid, “The Inhibition of 4-(2′-Amino-5′-methylphenylazo) antipyrine on Corrosion of Mild Steel in HCl Solution” Mater. Chem. Phys. 70 (2001) 268–273
F.M. Donahue, K. Nobe, “Theory of Organic Corrosion Inhibitors: Adsorption and Linear Free Energy Relationships”, J. Electrochem. Soc. 112 (1965) 886–891
E. Khamis, F. Bellucci, R. M. Latanision, E.S.H. El-Ashry, “Acid Corrosion Inhibition of Nickel by 2-(Triphenosphoranylidene) Succinic Anhydride”, Corrosion 47 (1991) 677–686
M. Soleimani, T. Kaghazchi, “The Investigation of the Potential of Activated Hard Shell of Apricot Stones as Gold Adsorbents”, J. Ind. Eng. Chem. 14 (2008) 28–37
U. Inel, D. Topalogln, A. Askin, F. Tumsek, “Evaluation of the Thermodynamic Parameters for the Adsorption of Some Hydrocarbons on 4A and 13X Zeolites by Inverse Gas Chromatography”, Chem. Eng. J. 88 (2002) 255–262
L. Warren, C.S. Julian, and P. Hariott, Unit Operations of Chemical Engineering, 4th ed., McGraw Hill, New York, 1985, p 217–219, 690
H. Armoosh, The Herbs in Book, 1st ed. House of the Gems, Damascus, 1999
Acknowledgments
One of the authors (A. M. Badiea) is thankful to Mr. Nasr Humaidy, deputy of PEPA and Dr. Ahmed Abdillah, Chairman of PEPA (Ministry of Oil, Yemen) for providing some financial support and to Ms. Marry (Düsseldorf, Germany) for her help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Badiea, A.M., Mohana, K.N. Corrosion Mechanism of Low-Carbon Steel in Industrial Water and Adsorption Thermodynamics in the Presence of Some Plant Extracts. J. of Materi Eng and Perform 18, 1264–1271 (2009). https://doi.org/10.1007/s11665-009-9378-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-009-9378-x