Abstract
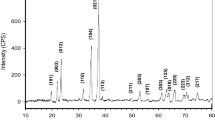
To minimize free carbon residue in the boron carbide (B4C) powder, a modified sol-gel process is performed where the starting materials as boric acid and citric acid compositions are adjusted. Because of boron loss in the form of B2O2(g) during the reduction reaction of the stoichiometric starting composition, the final B4C powders contain carbon residues. Thus, an excess H3BO3 is used in the reaction to compensate the loss and to obtain stoichiometric powders. Parameters of production have been determined using x-ray diffraction analysis and particle size analyses. The synthesized B4C powder using an excess boric acid composition shows no trace of carbon.





Similar content being viewed by others
References
D. Simeone, C. Mallet, P. Dubuisson, G. BaldiNozzi, and C.Gervais, Study of Boron Carbide Evolution Under Neutron Irradiation by Raman spectroscopy, J. Nuclear Mater., 2000, No. 277, p 1–10
G. de With, High Temperature Fracture of Boron Carbide: Experiments and Simple Theoretical Models, J. Mater. Sci., 1984, 19, 457-466
Z.L. Arbary and C.D. Reynolds, U.S. patent, No. 4,215,088, “Method for Fabricating Boron Carbide Articles”, July. 29, 1980
C.H. Jung, M.J. Lee, C.J. Kim, 2003 Preparation of Carbon Free B4C Powder from B2O3 Oxide by Carbothermal Reduction Process. Mater. Lett., 58(5): 609-614
E.G. Gray, Patent Specification, No. 687946, “A process for the Production of Boron Carbide”, Feb. 22, 1950
B.V.S. Subba Rao, A.D. Manohar, R.M. Rao, K. Somaraju, and R.K. Basu, Ceramic powder for high-tech-applications, Sangam Books, 1998, p 83–88
A. Sinha, T. Mahata, B.P. Sharma, Carbothermal Route for Preparation of Boron Carbide Powder from Boric Acid-Citric Acid Gel Precursor. J. Nuclear Mater., 2002, 301: 165-164
Carlsson M., Garcia-Garcia F.J., Johnsson M. 2002 Synthesis and Characterization of Boron Carbide Whiskers and Elongated Platelets. J. Crystal Growth, 236(3&4): 466-476
Thevenot F., 1990 Boron Carbide-A Comprehensive Review. J. Euro. Ceram. Soc., 74(6): 205–225
Alizadeh A., Taheri-Nassaj E., Ehsani N. 2004 Synthesis of Boron Carbide Powder by a Carbothermic Reduction Method. J. Euro. Ceram. Soc., 24(10-11): 3227-3234
B.V.S. Subba Rao, A.D. Manohar, R.M. Rao, K. Somaraju, and R.K. Basu, Ceramic powder for high-tech-applications, Sangam Books, 1998, p 82–83
Bose D.K., Nair K.U., Gupta C.K. 1986 Production of High Purity Boron Carbide. High Temp. Mat. Proc., 7(2&3): 133–140
Shi L., Gu Y., Chen L., Qian Y. 2003 A Low Temperature Synthesis of Crystalline B4C Ultrafine Powders. Solid State Commun., 128(1): 5-7
Mondal S., Banthia A.K. 2005 Low-Temperature Synthesis Route for Boron Carbide. J. Euro. Ceram. Soc., 25(2–3): 287-291
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hadian, A., Bigdeloo, J. The Effect of Time, Temperature and Composition on Boron Carbide Synthesis by Sol-gel Method. J. of Materi Eng and Perform 17, 44–49 (2008). https://doi.org/10.1007/s11665-007-9125-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-007-9125-0