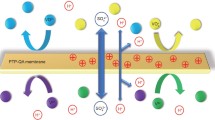
Abstract
This work reports the preparation and characterization of composite membranes with potential applications in flow battery devices. A polymer solution of polyvinylidene fluoride (PVDF), sulfonated polyether ether sulfone (SPEES), and polyether sulfone (PES) was used to prepare proton exchange membranes with low permeation of cationic species, which is a desirable feature in energy conversion devices, such as vanadium flow batteries (VRFB). Vanadyl ion (VO2+) acidic solution was selected as a model to reveal the permeation capacity through the membranes as an affordable alternative to the Nafion® 117 commercial membrane. The effects of the chemical composition and the thickness of the membranes on the morphology and proton exchange properties were evaluated. Characterization of the membranes was performed using physicochemical techniques including water uptake, swelling ratio, thermogravimetric analysis, scanning electron microscopy, Fourier transform infrared, surface charge density, atomic force microscopy, dynamic mechanical analysis, electrochemical impedance spectroscopy for proton conductivity, proton exchange rate, and the permeability of VO2+ ions. With VO2+ ion permeability of the order of 0.62 mM and H+ exchange rates of up to 498.5 × 1018 H+ cm−2*µm−1*min−1, the PVDF/PES/SPEES composite membranes emerge as an option with potential application for VRFB compared to the Nafion® 117 membrane, which presented a VO2+ ion permeation value of 2.72 mM and H+ exchange rates of 445.2 × 1018 H+ cm−2*µm−1*min−1.
Graphical Abstract









Similar content being viewed by others
References
C.N. Papadakis, M. Fafalakis, and D. Katsaprakakis, A Review of Pumped Hydro-Storage Systems. Energies 16(11), 4561 (2023).
C.R. Matos, P.P. Silva, and J.F. Carneiro, Overview of Compressed Air Energy Storage Projects and Regulatory Framework for Energy Storage. J. Energy Storage 55, 105862 (2022).
A. Berrada, A. Emrani, and A. Ameur, Life-Cycle Assessment of Gravity Energy Storage Systems for Large-Scale Application. J. Energy Storage 40, 102825 (2021).
S. Maddukuri, D. Malka, M.S. Chae, Y. Elias, S. Luski, and D. Aurbach, On the Challenge of Large Energy Storage by Electrochemical Devices. Electrochem. Acta 354, 136771 (2021).
K. Lourenssen, J. Williams, F. Ahnadpour, R. Clemmer, and S. Tasnim, Vanadium Redox Flow Batteries: A Comprehensive Review. J. Energy Storage 25, 100844 (2019).
P. Qian, H. Wang, L. Zhang, Y. Zhou, and H. Shi, An Enhanced Stability and Efficiency of SPEEK-Based Composite Membrane Influenced by Amphoteric Side-Chain Polymer for Vanadium Redox Flow Battery. J. Membr. Sci. 643, 120011 (2022).
S.J. Peighambardoust, S. Rowshanzamir, and M. Amjadi, Review of the Proton Exchange Membranes for Fuel Cell Applications. Int. J. Hydrog. Energy 35, 9349 (2010).
C.C. Yang and G.M. Wu, Study of Microporous PVA/PVC Composite Polymer Membrane and if Application to MnO2 Capacitors. Mater. Chem. Phys. 114, 948 (2009).
K. Fan, C. Wei, and J. Feng, Recent Progress of Mxene-Based Materials as Anodes in Sodium-Ion Batteries. J. Electron. Mater. 52, 847 (2023).
T. Wang, J.Y. Jeon, J. Han, J.H. Kim, C. Bae, and S. Kim, Poly(Terphenylene) Anion Exchange Membranes with High Conductivity and Low Vanadium Permeability for Vanadium Redox Flow Batteries (VRFBs). J. Membr. Sci. 598, 117665 (2020).
C.Y. Lee, C.A. Jiang, C.L. Hsieh, C.H. Chen, K.F. Lin, Y.M. Liu, and Y.P. Huang, Application of Flexible Integrated Microsensor to Internal Real-Time Measurement of Vanadium Redox Flow Battery. Sens. Actuators A Phys. 267, 135 (2017).
L. Ding, X. Song, L. Wang, Z. Zhao, and G. He, Preparation of Dense Polybenzimidazole Proton Exchange Membranes with Different Basicity and Flexibility for Vanadium Redox Flow Battery Applications. Electrochim. Acta 292, 10 (2018).
N. Kononenko, V. Nikonenko, D. Grande, C. Larchet, L. Dammak, M. Fomenko, and Y. Volfkovich, Porous Structure of Ion Exchange Membranes Investigated by Various Techniques. Adv. Colloid Interface Sci. 246, 196 (2017).
L. Kong, E. Palacios, X. Guan, M. Shen, and X. Liu, Mechanisms for Enhanced Transport Selectivity of Like-Charged Ions in Hydrophobic-Polymer-Modified Ion-Exchange Membranes. J. Membr. Sci. 658, 120645 (2022).
B. Jiang, L. Wu, L. Yu, X. Qiu, and J. Xi, A Comparative Study of Nafion Series Membranes for Vanadium Redox Flow Batteries. J. Membr. Sci. 510, 18 (2016).
Z. Fu, J. Liu, and Q. Liu, SPEEK/PVDF/PES Composite as Alternative Proton Exchange Membrane for Vanadium Redox Flow Batteries. J. Electron. Mater. 45, 666 (2015).
P. Kumar, S.K. Jagwani, and P.P. Kundu, A Study on the Heat Behaviour of PEM, Prepared by Incorporation of Crosslinked Sulfonated Polystyrene in the Blend of PVDF-co-HFP/Nafion, for its High Temperature Application in DMFC. Mater. Today Commun. 2, 1e (2015).
B.Y. García Limón, M.I. Salazar Gastelum, S.W. Lin, J.C. Calva-Yañez, and S. Perez-Sicairos, Preparation and Characterization of PVDF/PES/Nafion® 117 Membranes with Potential Application in Vanadium Flow Batteries. Rev. Mex. Ing. Quim. 18, 477 (2019).
H.J. Choi, C. Youn, S.C. Kim, D. Jeong, S.N. Lim, D.R. Chang, J.W. Bae, and J. Park, Nafion/Functionalized Metal–Organic Framework Composite Membrane for Vanadium Redox Flow Battery. Adv. Microporous Mesoporous Mater. 341, 112054 (2022).
Y. Wang, K. Feng, L. Ding, L. Wang, and X. Han, Influence of Solvent on Ion Conductivity of Polybenzimidazole Proton Exchange Membranes for Vanadium Redox Flow Batteries. Chin. J. Chem. Eng. 28, 1701 (2020).
V.I. Vlasov, N.A. Gvozdik, M.D. Mokrousov, S.V. Ryazantsev, S.Y. Luchkin, D.A. Gorin, and K.J. Stevenson, Ion-Exchange Membrane Impact on Preferential Water Transfer in all-Vanadium Redox Flow Battery. J. Power. Sources 540, 231640 (2022).
I.A. Prikhno, E.Y. Safronova, I.A. Stenina, P.A. Yurova, and A.B. Yaroslavtsev, Dependence of the Transport Properties of Perfluorinated Sulfonated Cation-Exchange Membranes on Ion-Exchange Capacity. Membr. Membr. Technol. 2, 265 (2020).
J. Yu, B. Yi, D. Xing, F. Liu, Z. Shao, Y. Fu, and H. Zhang, Degradation Mechanism of Polystyrene Sulfonic Acid Membrane and Application of its Composite Membranes in Fuel Cells. Phys. Chem. Chem. Phys. 5, 611 (2003).
A.L. Moster and B.S. Mitchell, Hydration and Proton Conduction in Nafion/Ceramic Nanocomposite Membranes Produced by Solid-State Processing of Powders from Mechanical Attrition. J. Appl. Polym. Sci. 113, 611 (2009).
T. Wang, S.J. Moon, D.S. Hwang, H. Park, J. Lee, S. Kim, Y.M. Lee, and S. Kim, Selective Ion Transport for a Vanadium Redox Flow Battery (VRFB) in Nano-Crack Regulated Proton Exchange Membranes. J. Membr. Sci. 583, 16 (2019).
M. Vijayakumar, B. Schwenzer, S. Kim, Z. Yang, S. Thevuthasan, J. Liu, G.L. Graff, and J. Hu, Investigation of Local Environments in Nafion-SiO2 Composite Membranes used in Vanadium Redox Flow Batteries. Solid State Nucl. Magn. Reson. 42, 71 (2012).
P. Sharma, S. Kumar, M. Bhushan, and V.K. Shahi, Ion Selective Redox Active Anion Exchange Membrane: Improved Performance of Vanadium Redox Flow Battery. J. Memb. Sci. 637, 119626 (2021).
J.A. Domínguez-Maldonado, O. García-Rodríguez, M. Aguilar-Vega, M. Smit, and L. Alzate-Gaviria, Reduction of Cation Exchange Capacity in a Microbial Fuel Cell and its Relation to the Power Density. Rev. Mex. Ing. Quim. 13, 527 (2014).
M. Jung, W. Lee, N.N. Krishnan, S. Kim, G. Gupta, L. Komsiyska, C. Harms, Y. Kwon, and D. Henkensmeier, Porous-Nafion/PBI Composite Membranes and Nafion/PBI Blend Membranes for Vanadium Redox Flow Batteries. Appl. Surf. Sci. 4501, 301 (2018).
S. Thangarasu and T.H. Oh, Progress in Poly(Phenylene Oxide) Based Cation Exchange Membranes for Fuel Cells and Redox Flow Batteries Applications. Int. J. Hydrog. Energy 46, 38381 (2021).
J. Xi, Z. Wu, X. Qiu, and L. Chen, Nafion/SiO2 Hybrid Membrane for Vanadium Redox Flow Battery. J. Power. Sources 166, 531 (2007).
E. Sgreccia, J.F. Chailan, M. Khadhraoui, M.L. Di Vona, and P. Knauth, Mechanical Properties of Proton-Conducting Sulfonated. J. Power. Sources 195, 7770 (2010).
W. Mabrouk, K. Charradi, A. Mellekh, A. Hafiane, Q.A. Alsulami, H.M. Meherzi, R. Chtourou, and S.M. Keshk, Enhanced Proton Conductivity of a Sulfonated Polyether Sulfone Octyl Sulfonamide Membrane via the Incorporation of Protonated Montmorillonite. J. Electron. Mater. 52, 2158 (2023).
Acknowledgments
The authors thank Tecnológico Nacional de México/ Instituto Tecnológico de Tijuana (grant no. 18326.23-P) for providing materials and equipment for this work. B.Y. Garcia-Limón and L.J. Salazar-Gastélum thank the Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCyT, Mexico) for providing the scholar fellowship for their Ph.D. studies. The authors also thank Victor A. Reyes-Villegas, M.Sc., for his comments to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
García-Limón, B.Y., Salazar-Gastélum, L.J., Salazar-Gastélum, M.I. et al. Composite Membranes of PVDF/PES/SPEES for Flow Battery Applications. J. Electron. Mater. (2024). https://doi.org/10.1007/s11664-024-11011-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11664-024-11011-1