Abstract
4-Methylbenzylammonium hydrogen succinate (4MLBAHS) was synthesized and successfully grown via a slow solvent evaporation process. The structure was solved and the space group identified as \({\text{P}}\overline{1 }\). In the 4MLBAHS molecule, 4-methylbenzylammonium and hydrogen succinate are linked by O–H···O and N–H···O bonds. Functional group vibrations were revealed through vibrational analysis. UV–Vis–NIR analysis was used to determine the transparency bandwidth range from 260 nm to 1100 nm. 1H and 13C nuclear magnetic resonance (NMR) spectroscopy established the carbon–hydrogen network in 4MLBAHS. In 1H NMR, the chemical shift for the NH2 group of 4-methylbenzylamine was revealed at 1.42 ppm. This was shifted to 8.094 ppm in 4MLBAHS, owing to the origination of the N–H···O bond between the 4-methylbenzylammonium cation and hydrogen succinate anion. The third-order nonlinear optical characteristics were analysed using Z-scan analysis. χ3 was established as 3.0879 × 10−5 esu. The presence of hydrogen bond interactions in the 4MLBAHS crystal enhanced the χ3 value.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Materials having optical nonlinearity with good physical and chemical flexibility are critical for a variety of applications. Novel materials have been utilized in the development of optical communication systems, where organic materials have significant potential for improving the systems.1,2 Organic materials are widely used due to their high figure of merit in linear and nonlinear optical response during device fabrication.3 Organic crystals offer synthetic flexibility for the design and production of higher-order nonlinear optical (NLO) materials used in optical switching applications, optical information processing and storage, and laser technology.4,5
Carboxylic acid has been found to be a potential component in the growth process owing to its strong hydrogen bond interactions with the reacting substance. Succinic acid exists in the neutral state as succinic acid and in the ionized state as succinate, forming a strong intermolecular hydrogen bond with other molecules. It is used extensively in the pharmaceutical and food industries, in the fabrication of high-electron-mobility transistors, and for the production of biodegradable polymers and surfactants.6,7,8 Carboxylic acids, when associated with an amine group, give a higher-order nonlinear response.9 The slow evaporation solution growth process is simple and economical, and can be carried out at low temperature.10 Our researchers have worked on efficient organic NLO compounds under the amino acid category, such as L-alaninium succinate (LAS) and valinium succinate (LVS). Both crystals show wide optical transmittance, which enhances their NLO properties.11,12 We have also reported several new 4-methylbenzylamine-based crystals.13,14,15,16,17,18,19,20 Recently, new crystals showing enhanced third-order NLO efficiency and with wide optical transparency have been developed and reported.21,22,23
All these investigations demonstrate that 4-methylbenzylamine plays a prominent role in the field of photonics. In this work, the structural, spectral and optical properties of 4-methylbenzylammonium hydrogen succinate (4MLBAHS) are reported. In the 4MLBAHS crystal, succinic acid is a carboxylic acid with the potential to form strong hydrogen bond interactions with 4-methylbenzylamine, forming a new structure. The crystal reveals a two-dimensional structure between the cation and anion connected by hydrogen bonds. 4MLBAHS can be utilized in night vision devices and in optical-limiting and photonic device applications.
Materials and Methods
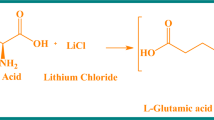
Analytical reagent (AR)-grade solution of 4-methylbenzylamine (4MLBA) and succinic acid (SA) were used for synthesizing the title crystal 4MLBAHS [(C8H12N+)(C4H5O4−)] by the slow evaporation solution growth method. 4MLBAHS was developed by mixing 4MLBA and SA in equimolar proportions. The mixture was stirred in 50 mL of deionized water for 3 h, and the resulting solution was kept undisturbed. Transparent crystals developed due to spontaneous nucleation and were harvested after 15 days. The crystal growth reaction of 4MLBAHS is given in Fig. 1.
Results and Discussion
Single-Crystal X-ray Diffraction (SCXRD) Analysis
Unit cell constants were estimated using a Bruker APEX3 diffractometer. The observed cell constants are a = 9.3710(4) Å, b = 12.0713(5) Å, c = 22.5779(10) Å, α = 94.959(2)°, β = 90.964(2)°, γ = 96.082(19)° and V = 2529.26(19) Å3. The structure was solved using SHELX-97, and using the full-matrix least-squares method, refinement was performed to an R-value of 0.1680. A molecular diagram was generated using Mercury 3.8 software. The 4MLBAHS crystal data are given in Supplementary Table S1. The interatomic distances and angles of 4MLBAHS are presented in Supplementary Table S2 and Table S3. The 4MLBAHS structure contains (C8H12N)+ cation and hydrogen succinate (C4H5O4)− anion joined by N(1)–H(1C)···O(6) bonds. The anion molecules are connected with neighbouring anions through O(4)–H(4)···O(10) hydrogen bonds. Hydrogen bonds are shown in Supplementary Table S4. A two-dimensional structure arises because of the weak C–H···π interactions. In the structure of 4MLBAHS, the methylene unit (CH2) from the positively charged ammonium group (C8H12N)+ is a donor and the hydrogen succinate (C4H5O4)− negatively charged group acts as an acceptor. An ORTEP (Oak Ridge Thermal Ellipsoid Plot) diagram of 4MLBAHS is given in Fig. 2a. Figure 2b shows the crystal packing diagram of 4MLBAHS viewed along the b*-axis. The morphology of the grown crystals was indexed using WinXMorph software. Figure 2c shows the morphology of 4MLBAHS. It contains 14 well-developed faces (1 0 0), (1 0 −1), (−1 0 0), (−1 0 1), (0 1 0), (0 1 −1), (0 −1 0), (0 −1 1), (−1 1 0), (−1 1 −1), (1 −1 1), (1 1 0), (0 0 1) and (0 0 −1). The growth rate of 4MLBAHS is extended along the crystallographic a-axis. The structure of 4MLBAHS is deposited as CCDC number 2233050.
Linear Optical Analysis
The optical transmission spectrum of the grown organic crystal of 4MLBAHS was recorded with a PerkinElmer ultraviolet–visible–near-infrared (UV–Vis–NIR) spectrophotometer as shown in Fig. 3. 4MLBAHS exhibits high transparency, with a cut-off wavelength of 260 nm. The wide optical transmission in the 260–1100 nm region reveals that 4MLBAHS possesses fewer structural defects and can be used in optical applications.17 When compared with the previously reported crystals of 4-methylbenzylammonium oxalate hydrate (4MLBAOX) and 4-methylbenzylammonium hydrogen maleate (4MBAHM), their transmission range is from 262 nm to 1100 nm,21,23 whereas the 4MLBAHS crystal possesses a more prominent wide transmission window and can be utilized in NLO device applications.
Fourier transform infrared (FTIR) and FT- Raman Vibrational Studies
The vibrational spectra of the 4MLBAHS crystal are given in Fig. 4a and b. The peak appearing at around 3360 cm−1 is due to NH3+ stretching. NH3+ symmetric bending modes were identified at 1610 cm−1 and 1644 cm−1 in IR and 1578 cm−1 and 1615 cm−1 in Raman spectroscopy. COO− asymmetric and symmetric stretching of SA was observed at 1514 cm−1 and 1412 cm−1 in IR and 1414 cm−1 in Raman spectroscopy. COO− bending was found at 639 cm−1 and 642 cm−1 in IR and Raman spectroscopy, respectively. The presence of NH3+ and COO− vibrations ensures the formation of the 4MLBAHS crystal. The vibrations of the other functional groups of 4MLBAHS were analysed in accordance with the literature17,20,24,25,26,27,28 and are presented in Table I.
1H NMR Spectral Study
1H and 13C NMR spectra of 4MLBAHS were recorded with a Bruker 400 MHZ spectrometer, and the spectra are depicted in Fig. 5a and b, respectively. Dimethyl sulfoxide (DMSO) was used as water (peak at 3.432 ppm) and DMSO solvent was observed at 2.506 ppm.18
In pure 4MLBA, the signals for CH3, CH2, CH (H in aromatic ring) and NH2 groups were recorded at 2.320 ppm, 3.795 ppm, 6–8 ppm and 1.42 ppm, respectively. In 4MLBAHS, the signals for CH3, CH2 and CH (H in aromatic ring) were observed at 2.087 ppm, 3.982 ppm and 7.220−7.350 ppm, respectively.19,24 Part of the spectrum appears as a quartet, and the coupling constant is given as J (0.02 ppm × 400.231 MHz) = 8.00462 Hz. The observed intensities show that the inner peaks are taller than the outer peaks, such as (7.239 ppm and 7.330 ppm) representing inner peaks and (7.220 ppm and 7.350 ppm) representing outer peaks. The resonating protons are coupled to form a strong coupling effect, leading to variation in the splitting pattern of the peaks known as the ‘roof effect’.29 The chemical shift for NH2 is largely shifted and observed at 8.094 ppm. This is attributed to the protonation of the NH2 group and ensures the development of the title crystal.30 In succinic acid, the signal for CH2 is revealed at 2.425 ppm, and in 4MLBAHS it is observed at 2.312 ppm.12
13C NMR Spectral Study
In 13C NMR, for pure 4MLBA, the signals for CH3, CH2 and CH (H in aromatic ring) are revealed at 20.97 ppm, 46.21 ppm and 126.95–140.47 ppm, respectively. In 4MLBAHS, the signals for CH3, CH2 and CH (H in aromatic ring) are shifted and observed at 20.23 ppm, 42.81 ppm and 128.83–139.56 ppm.30 In succinic acid, the CH2 and COOH signals are revealed at 28.75 ppm and 173.48 ppm. In 4MLBAHS, the signals are shifted and observed at 31.23 ppm and 179.57 ppm, respectively.31,32 The intermolecular interactions, molecular structure and protonation of the amine group were well established by the shifts observed in both 1H and 13C NMR spectra. The shifts are tabulated along with the assignments in Table II.
Z-Scan Analysis
Z-Scan was carried out at 632.8 nm using a He-Ne laser (intensity = 3.13 mW cm−2). Figure 6a and b depicts the closed and open aperture graphs of 4MLBAHS. The observed n2, β and χ3 values are displayed in Table III. The pre-focal peak followed by post-focal valley reveals the self-defocusing nature of the 4MLBAHS crystals, making them appropriate for use in optical sensors and night vision devices.33,34,35 4MLBAHS shows minimum transmittance at Z = 0 due to the reverse saturable absorption in the open aperture curve. This can be effectively employed in optical limiting applications.36,37 The χ3 value of 4MLBAHS is found to be 3.0879 × 10−5 esu, which is higher than the χ3 values of LMSA, KSSA, MA-KDP, OA-KDP and BCSSA crystals reported in previous works, which are compared and shown in Table IV. The hydrogen bonds in the 4MLBAHS molecule improve the charge transfer mechanism, which results in a higher χ3 value.
Conclusion
Organic crystals of 4MLBAHS were effectively synthesized by a slow evaporation technique. The structure was solved and comprises 4-methylbenzylammonium (C8H12N)+ cation and hydrogen succinate (C4H5O4)− anion connected by hydrogen bonds. 4MLBAHS is transparent from 260 nm to 1100 nm. The functional group vibrations were analysed. The molecular structure and the significance of intermolecular hydrogen bonding were established by NMR spectral analysis. 4MLBAHS exhibits self-defocusing and reverse saturable absorption properties. The χ3 value of the crystal is 3.0879 × 10−5 esu. The results reveal that 4MLBAHS can be utilized in night vision, optical-limiting and photonic devices.
Data Availability
All data are presented in the manuscript. Crystallographic data have been deposited at the Cambridge Crystallographic Data Centre, CCDC number CCDC2233050.
References
L. Jiang, H. Dong, and W. Hu, Organic single crystal field-effect transistors: advances and perspectives. J. Mater. Chem. 20, 4957 (2010).
S.L. Childs, P.A. Wood, N. Rodriguez-Hornedo, L.S. Reddy, and K.I. Hardcastle, Analysis of 50 crystal structures containing carbamazepine using the materials module of mercury CSD. Cryst. Growth Des. 9, 1869 (2009).
B. Babu, J. Chandrasekaran, R. Thirumurugan, V. Jayaramakrishnan, and K. Anitha, Experimental and theoretical investigation on 2-amino 5-bromopyridinium L-tartrate-A new organic charge-transfer crystal for optoelectronics device applications. J. Mater. Sci. Mater. Electron. 28, 1124 (2017).
J.H. Kleinwiele, M.A. Bader, I. Baker, S. Soria, P. Sirror, and G. Marousky, Ablation dynamics of periodic nanostructures for polymer-based all-optical devices. Synth. Met. 127, 53 (2002).
H.H. Fang, J. Yang, J. Feng, T. Yamao, S. Hotta, and H.B. Sun, Functional organic single crystals for solid-state laser applications. Laser Photonics Rev. 8, 687 (2014).
P. Rajesh and P. Ramasamy, A study on optical, thermal, mechanical, dielectric, piezoelectric and NLO properties of unidirectional ammonium chloride added ammonium dihydrogen phosphate crystal. Mater. Lett. 63, 2260 (2009).
J.B. McKinlay, C. Vieille, and J.G. Zeikus, Prospects for a bio-based succinate industry. Appl. Microbiol. Biotechnol. 76, 727 (2007).
L. Agarwal, J. Isar, and R.K. Saxena, Rapid screening procedures for identification of succinic acid producers. J. Biochem. Biophys. Methods 63, 24 (2005).
S. Krishnan, C. Justin Raj, and S. Jerome Das, Growth and characterization of novel ferroelectric urea–succinic acid single crystals. J. Cryst. Growth 310, 3313 (2008).
R.J. Davey, The role of the solvent in crystal growth from solution. J. Cryst. Growth 76, 637 (1986).
C. Ramachandra Raja, G. Gokila, and A. Antony Joseph, Growth and spectroscopic characterization of a new organic nonlinear optical crystal: L-Alaninium succinate. Spectrochim. Acta Part A 72, 753 (2009).
C. Ramachandra Raja and A. Antony Joseph, Crystal growth and comparative studies of XRD, spectral studies on new NLO crystals: L-Valine and L-valinium succinate. Spectrochim. Acta Part A 74, 825 (2009).
R. Aarthi, A. Thiruvalluvar, and C. Ramachandra Raja, 4-Methylbenzylammonium chloride hemihydrate. IUCr Data 2, x171213 (2017).
R. Aarthi, A. Thiruvalluvar, and C. Ramachandra Raja, (4-Methylphenyl) methanaminium bromide hemihydrate. IUCr Data 3, x180270 (2018).
R. Aarthi, A. Thiruvalluvar, and C. Ramachandra Raja, Bis(4-methylbenzylammonium) tetrabromidozincate. IUCrData 3, x180648 (2018).
R. Aarthi, A. Thiruvalluvar, and C. Ramachandra Raja, catena-Poly[bis(4-methylbenzylammonium) [[dibromidocadmate(II)]-di-μ-bromido]]. IUCr Data 3, x180780 (2018).
R. Aarthi and C. Ramachandra Raja, Spectral and optical characterization of the new semi-organic crystal: 4-methylbenzylammonium chloride hemihydrate, to establish protonation and the effect of resultant hydrogen bonding. Bull. Mater. Sci. 209, 1 (2019).
R. Aarthi and C. Ramachandra Raja, Synthesis, structural, spectral and optical studies of (4-methylphenyl) methanaminium bromide hemihydrate: a new semiorganic crystal for laser applications. J. Mater. Sci. Mater. Electron. 30, 6947 (2019).
R. Aarthi and C. Ramachandra Raja, Confirmation of supramolecular structure and its impact on nonlinear optical properties of Bis(4-methylbenzylammonium) tetrabromido zincate: a new semiorganic crystal. J. Mol. Struct. 1189, 338 (2019).
R. Aarthi and C. Ramachandra Raja, Probing the structure–property relationship of a new semiorganic nonlinear optical crystal: catena-poly[bis(4- methylbenzylammonium) [[dibromidocadmate(II)]-di-l-bromido. J. Mater. Sci. Mater. Electron. 30, 8698 (2019).
P. Deepa, R. Aarthi, S. Kalainathan, and C. Ramachandra Raja, Synthesis, structural, spectral and third-order nonlinear optical analysis of solution–grown 4-methylbenzylammonium oxalate hydrate: a novel organic crystal for laser applications. J. Mater. Sci. Mater. Electron. 34, 925 (2023).
P. Deepa, R. Aarthi, S. Kalainathan, and C. Ramachandra Raja, Synthesis, structural, spectral and optical analysis of a novel semi-organic nonlinear optical crystal: Bis(4-methylbenzylammonium)hydrogen phosphate monohydrate. Opt. Quantum Electron. 55, 758 (2023).
P. Deepa, R. Aarthi, S. Kalainathan, and C. Ramachandra Raja, Interpretation of structural and spectroscopic characteristics of 4-methylbenzylammonium hydrogen maleate (4MBAHM): a novel organic crystal. Opt. Quantum Electron. 55, 1124 (2023).
R. Aarthi, P. Umarani, and C. Ramachandra Raja, Molecular structural confirmation and influence of hydrogen bond on third order nonlinear properties of Bis(4-methylbenzylammonium) Tetra chloridocadmate (II) single crystal. Optik 164, 449 (2018).
B.K. Singh, N. Sinha, N. Singh, K. Kumar, M.K. Gupta, and B. Kumar, Structural, dielectric, optical and ferroelectric property of urea succinic acid crystals grown in aqueous solution containing maleic acid. J. Phys. Chem. Solids 71, 1774 (2010).
I. Sivagami, R.M. Jauhar, P. Era, V. Viswanathan, G. Vinitha, S. Ranjani, and T. Sivanesan, Growth, structural, spectral, Hirshfeld analysis, photoluminescence, linear and third order NLO properties of a novel organic p-toluidinium succinate succinic acid single crystal. J. Cryst. Growth 580, 1 (2022).
G. Harry Brittain, Vibrational spectroscopic studies of cocrystals and salts. Cocrystal products formed by benzylamine, α-methylbenzylamine, and their chloride salts. Cryst. Growth Des. 11, 2500 (2011).
A. Arunkumar, P. Ramasamy, K. Vishnu, and M.K. Jayaraj, Growth, structural, thermal, optical, and electrical properties of potassium succinate–succinic acid crystal. J. Mater. Sci. 49, 3598 (2014).
B. Luy, Adiabatic z-filtered J-spectroscopy for absorptive homonuclear decoupled spectra. J. Magn. Reson. 201, 18 (2009).
R. Aarthi, P. Umarani, and C. Ramachandra Raja, Interpretation of molecular structure and third-order nonlinear optical studies of 4-methylbenzylammonium nitrate single crystal. Appl. Phys. A 124, 1 (2018).
L.B. Landim, E.O. Miranda Jr, N.A. de Araujo, J.C. Pinto, E.C.M. Cabral-Albuquerque, S. Cunha, and R.L. Fialho, Solvent-free mechanochemical polymerization of urea-succinic acid and urea-succinic acid-glycerol mixtures. J. Clean. Prod. 238, 1 (2019).
J.S. Clawson, F.G. Vogt, J. Brum, J. Sisko, D.B. Patience, W. Dai, S. Sharpe, A.D. Jones, T.N. Pham, M.N. Johnson, and R.C.P. Copley, Formation and characterization of crystals containing a pleuromutilin derivative, succinic acid and water. Cryst. Growth Des. 8, 4120 (2008).
M. Sheik-Bahae, A.A. Said, T.H. Wei, D.J. Hagan, and E.W. Van Stryland, Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 26, 760 (1990).
M. Sheik-bahaei, P. Mukherjee, and H.S. Kwok, Two-photon and three-photon absorption coefficients of InSb. J. Opt. Soc. Am. B 3, 379 (1986).
M. Nageshwari, P. Jayaprakash, C.R. Kumari, G. Vinitha, and M.L. Caroline, Growth, spectral, linear and nonlinear optical characteristics of an efficient semiorganic acentric crystal: L-valinium L-valine chloride. Phys. B Condens. Matter. 511, 1 (2017).
S. Raghavendra, T.C. Shetty, C.C. Kumar, S.R. Maidur, P.S. Patil, C.K. Quah, G.S. Ananthnag, S. Chandraju, and S.M. Dharmaprakash, Nonlinear reverse saturation absorption, self-defocussing behaviour and structure-property relationship of a novel 2,3,4- trimethoxy-4′-nitrochalcone single crystal. J. Mol. Struct. 1193, 177 (2019).
S. Raghavendra, Chandraju Sadolalu Chidankumar, Arasalike Jayarama and Sampyady Medappa Dharmaprakash, 1-[4-(methylsulfanyl) phenyl]-3-(4-nitropshenyl) prop-2-en-1-one: a reverse saturable absorption based optical limiter. Mater. Chem. Phys. 149, 487 (2015).
M. Nageshwari, C.R. Kumari, G. Vinitha, M.P. Mohamed, S. Sudha, and M.L. Caroline, Crystal growth, structural, spectral, thermal, dielectric, linear and nonlinear optical characteristics of a new organic acentric material: L-Methionine Succinic acid (2/1). J. Mol. Struct. 1155, 101 (2018).
M. Anis, G.G. Muley, A. Hakeem, M.D. Shirsat, and S.S. Hussaini, Exploring the influence of carboxylic acids on nonlinear optical (NLO) and dielectric properties of KDP crystal for applications of NLO facilitated photonic devices. Opt. Mater. 46, 517 (2015).
R. Gomathi, S. Madeswaran, and D. Rajan Babu, Bulk growth, electrical, linear, third order nonlinear optical and optical limiting properties on bis(cyclohexylammonium) succinate succinic acid crystal. Mater. Chem. Phys. 207, 84 (2018).
Acknowledgments
The authors thank the Sophisticated Analytical Instruments Facility (SAIF), Indian Institute of Technology, Chennai, for providing SCXRD and Raman spectroscopy. The authors gratefully acknowledge the Instrumentation Centre of St. Joseph’s College, Trichy for recording UV–Vis–NIR and FTIR spectra, and Gandhigram University, Dindigul, for providing the NMR spectrum. Also, the authors express special thanks to Dr. D. Sastikumar, NIT, Trichy, for providing us with a third-order nonlinear testing facility for recording Z-scan measurements.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
PD: crystal growth, characterization and manuscript writing. RA: formal analysis. SK: structure determination. CRR: supervision.
Corresponding author
Ethics declarations
Conflict of interest
We wish to confirm that there are no conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Ethical Approval
Not applicable
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deepa, P., Aarthi, R., Kalainathan, S. et al. Investigation on the Structure, Spectral and Third-Order Nonlinear Optical Analysis of a New Organic Crystal: 4-Methylbenzylammonium Hydrogen Succinate. J. Electron. Mater. 53, 2333–2339 (2024). https://doi.org/10.1007/s11664-024-10992-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-024-10992-3