Abstract
In this study, the effects of gamma radiation with doses ranging from 0 KGy to 100 KGy on the structural and optical properties of the Beta Metal-free Phthalocyanine (β-H2Pc) powder and films were investigated. X-ray diffraction and scanning electron microscope were used to examine the crystalline and morphological structures of the thin films. While Fourier transform infrared and absorption spectra were utilized to study the changes in molecular structure and energy absorption, respectively. The results showed that while γ-irradiation induced changes in the surface morphology and optical properties of β-H2Pc, the molecular structure remained stable except for the disappearance of the hydroxyl (OH) functional group peak. The irradiation dose was found to affect the dielectric constants, dispersion characteristics, and nonlinear optical susceptibility of the material. The β-H2Pc thin films exhibited high absorption coefficients and refractive index values, making them promising candidates for optoelectronic devices such as solar cells. However, careful consideration of the effects of gamma irradiation on other properties of β-H2Pc such as stability, toxicity, and cost-effectiveness is necessary.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal-free phthalocyanines (H2Pcs) are large aromatic organic compounds that are constructed of four isoindole units linked by nitrogen atoms.1 (H2Pcs) have 18 delocalized π electrons that characterize their strong absorption in the visible spectrum region. H2Pcs have similar chemical and thermal stability as metal phthalocyanines but are more environmentally friendly and easier to synthesize.2 Studies have shown that H2Pc films exhibit excellent gas sensing performance for various gases such as NO2, NH3, and H2S, and thus they have potential applications in gas sensing.3 Furthermore, H2Pc has been used in organic photovoltaic cells as a donor material, due to its high electron mobility and strong absorption in the visible region.4 H2Pc also shows encouraging potential in the field of optoelectronics as a semiconducting material for various devices, such as organic thin-film transistors,5 light-emitting diodes,6 organic field-effect transistors, optical sensing7 and solar cells.8 Additionally, H2Pc has been investigated as a potential candidate for photodynamic therapy due to its strong absorption in the near-infrared region and high singlet oxygen generation efficiency.
However, their stability under high radiation environments is still an area of active research. Gamma irradiation can have various effects on phthalocyanines, including changes in their optical properties, reduction in thermal stability, alterations in chemical and physical properties, and induction of degradation and damage. Gamma irradiation can shift the absorption peaks of phthalocyanines to shorter or longer wavelengths or create new peaks, which can affect the material's light absorption ability, making it useful in certain applications, such as photovoltaic device design. However, it can also reduce the material's thermal stability, alter its morphology, crystallinity, and molecular weight, and induce degradation and damage, which can lead to a decrease in its performance and functionality over time.
Correspondingly, it has been shown that H2Pc thin films can be affected by high energy radiation, which can alter their physical properties and create defects and vacancies.9 Gamma irradiation can cause changes in the morphology, structure, and optical properties of H2Pc thin films, which can affect their behavior in radiation environments. Studies have shown that gamma irradiation can lead to the formation of defects and vacancies in H2Pc thin films, which can alter their electronic and optical properties. The degree of impact depends on the irradiation dose, with higher doses causing more significant changes. Despite these effects, H2Pc thin films have been found to retain their stability and high-quality morphology after irradiation, indicating their potential for use in optoelectronic and sensing applications under harsh radiation environments. There are several examples of the harsh environments such as nuclear power plants, reactor dismantling, nuclear waste storage, space, and high-energy physics facilities.10
These findings suggest that gamma irradiation can be a useful tool for tuning the properties of H2Pc thin films for specific applications in radiation environments. Therefore, understanding the effects of gamma irradiation on H2Pc films can provide insights into their behavior in harsh radiation environments and inform the development of new applications in optoelectronics and sensing. Overall, the effects of gamma irradiation on phthalocyanines can be both beneficial and detrimental, depending on the specific application and the desired properties of the material. Therefore, careful consideration of the irradiation conditions and their effects is necessary for the optimal utilization of phthalocyanines in various applications.
The impact of gamma irradiation on the physical properties of various phthalocyanine derivatives in the form of thin films has been widely studied. Yaghmour11 investigated the effect of gamma irradiation on MnPc thin films at doses of 50 kGy and 150 kGy and observed changes in their optical properties. They found that the optical bandgap of the MnPc thin films decreased with increasing gamma irradiation dose, indicating an increase in the conjugation length and a decrease in the energy required for electron excitation. Darwish et al.12 examined the structural and optical properties of NiPc thin films at absorbed doses of 100 kGy, 200 kGy, and 300 kGy. Gamma irradiation caused a decrease in the crystallite size and an increase in the dislocation density of the NiPc thin films. A shift in the absorption edge towards lower energies was also observed, indicating a decrease in the bandgap energy. Also, the impact of gamma irradiation on the structural and optical properties of many other metallophthalocyanine (MPc) such as SnPc,13 CoPc,14 AlPcCl,15 SiPcCl216 and GaPcCl17 were studied. All of these studies have shown that exposure to gamma radiation can induce changes in the optical and structural properties of thin films.
To the best of our knowledge, detailed studies on the influences of gamma irradiation on the optical characterizations of β-H2Pc prepared by thermal deposition technique have not been extensively reported. The objective of the current work is to prepare nanocrystalline β-H2Pc thin films by using the thermally evaporated deposition method and to investigate the effect of γ-irradiation with various doses on their structural and optical features (linear and nonlinear) for employment as a potential material in optoelectronic devices.
Experimental Details
Thin Film Preparation and Physical Characterizations
Beta Metal-free Phthalocyanine (β-H2Pc) powder was obtained from Eastman Kodak, USA, and used as received. Thin films of β-H2Pc were deposited onto quartz and glass substrates using the thermal evaporation method (Edwards Co., model E306-A, England) under high vacuum conditions (10−4 Pa) for optical and structural measurements, respectively. The β-H2Pc powder was heated slowly in a molybdenum boat to deposit the thin films, while the substrate temperature was kept at room temperature. The film thickness (152 nm) and deposition rate (2.5 Å/s) were controlled using a quartz crystal thickness monitor (Edwards Co., model FTM6, England) attached to the work chamber.
The β-H2Pc powder and thin films were exposed to γ-rays using a 60Co irradiator chamber (Indian cell GC 4000 Å) at room temperature. The samples were placed in a drawer and irradiated with a dose rate of 10 kGy/100 min, with total doses ranging from 20 kGy to 100 kGy. The thin films were exposed for varying durations to achieve a series of different integrated absorbed doses. The pristine β-H2Pc thin film was irradiated at the National Center for Radiation Research and Technology (NCRRT) in Cairo, Egypt.
The obtained x-ray diffraction tracings (XRD) were used to study the structural properties. A Shimadzu x-ray diffractometer (XRD 6000) with a monochromatic CuKα radiation target and Ni filter (λ = 0.15406 nm) was used for the characterizations. The x-ray tube was operated at a voltage of 40 kV and a current of 30 mA. The diffraction tracings were recorded at a scanning speed of 8°/min in the normal 2θ range of 4° to 90°. The morphology of the samples was examined using the scanning electron microscope (JEOL JSM-636 OLA, Japan) at potential 15 kV and 20 kV. The particle size distribution was obtained using the image-J software program.
The Fourier transform infrared (FTIR) spectrum of β-H2Pc has been obtained on a Bruker (Alpha II) spectrophotometer equipped with a Platinum diamond ATR module. The spectral resolution was 2 cm−1. The spectrum was recorded in the 400−1–4000 cm−1 region at room temperature. The photoluminescence (PL) spectra of the samples were obtained using a Cary Eclipse fluorescence spectrophotometer. The spectra were recorded in the wavelength range of 300 nm–900 nm, and the scan rate was 600 nm/min.
To measure the reflectance, absorbance, and transmittance of the β-H2Pc thin films, a double-beam spectrophotometer (JASCO Co., model V-670, Japan) was used. The spectral range used for the measurements was 190 nm–2500 nm. The absorption and transmission scans were carried out with the films placed at a normal incidence of light and a blank quartz substrate as a reference. The reflection scan was conducted at an incident angle of 5° using an Al-mirror as a reference.
Method of Calculation
The preferred orientation of β-H2Pc thin films was evaluated by the texture coefficient (TC) parameter according to the following expression18:
where \({I}_{0(hkl)}\), and \({I}_{(hkl)}\) denote the standard and the measured relative intensities of the individual crystal planes (hkl), and N represents the number of the most intens peaks. The standard peak intensity for the reflection (hkl) was taken from the JCPDS card No. 37-1844.
The mean crystallite size (D) of β-H2Pc thin films was estimated to check the nanostructure using Debye–Scherrer’s formula. Also, other microstructural parameters such as the microstrain (ε), the average dislocation density (δ), and the number of crystallites per unit surface area (χ) were obtained from the full width at half maximum of highly intense peak (β) in radian using the following relations17:
where λ is the x-ray wavelength, θ is the diffraction angle, and d is the film thickness.
The absolute values of transmittance, T(λ), and reflectance, R(λ), are taken from the spectrophotometer and were corrected for canceling the absorbance and reflectance of the quartz substrate using a computer software package based on the subsequent relations19:
where Ift and Iq are the intensities of light passing through the thin film-quartz system and the reference quartz, respectively, and Rq is the reflectance of the quartz substrate.
where Ifr and Im are the intensities of the reflected light from the sample and the Al-mirror reference, respectively, and Rm is the Al-mirror reflectance.
The absorption coefficient, α, the absorption index, k, and the refractive index, n, of the thin films can be estimated from the absolute values of T(λ) and R(λ) using a computer program according to the following equation20
The value of energy gap Eg and electronic transition type can be determined using the following formula21:
where B is a constant and s is an exponent used for identifying the transition.
The main Dispersion parameters are completely described through the Wemple and DiDomenico approximation in terms of a single oscillator model according to the following expression22:
where, Ed, Eo and E are the dispersion energy, the oscillator energy, and the photon energy, respectively.
The refractive index can be expressed as a function of the wavelength by the following Eq. 23
where εL is the high-frequency lattice dielectric constant, N/m* is the ratio of the free carrier concentration to the effective mass, e is the elementary electronic charge, εo is the permittivity of free space, and c is the speed of light.
The imaginary ε2 and real ε1 parts of the complex dielectric constant are determined using the following relations24:
The dielectric loss tangent or the dissipation factor tan(δ) is evaluated by the following expression25:
Both the volume energy loss function (VELF) and surface energy loss function (SELF) is given by the following Eq. 26:
The real part σ1 and the imaginary part σ2 of the complex optical conductivity are given by27:
where ω is the angular frequency.
Miller developed a simple relation that relates the third-order nonlinear optical susceptibility χ(3) to the static refractive index n0 through the following relation28:
Also, the nonlinear refractive index (n2) is evaluated according to Miller’s rule by using the following formula29:
Results and Discussion
Structural and Surface Morphology Analysis
The XRD pattern of β-H2Pc in powder form was studied in Fig. 1a to investigate the effect of γ-irradiation with different dosages. The picture showed a polycrystalline nature with different preferred orientations. A hump was observed in the pattern at higher γ-irradiation doses. This hump could be attributed to the formation of defects or imperfections in the crystal structure of the powder.30 These defects could be due to the creation of lattice defects and atomic vacancies that can alter the crystal structure of the powder upon gamma irradiation. The defects generated in the β-H2Pc powder may cause changes in its electronic and optical properties, which can have significant consequences for its applications.
The XRD pattern of β-H2Pc film subjected to γ-irradiation with different doses is presented in Fig. 1b The film exhibited a preferential orientation in the (001) direction with a hump of amorphism, indicating that it belongs to a nano-sized structure. The presence of the diffraction hump confirms the amorphous nature of the film. The position of the (001) peak was not affected by changing the γ-irradiation doses, but its intensity decreased with increasing gamma dose. While the hump of amorphism increased. The decrease in the intensity of the (001) peak with increasing gamma dose can be attributed to the radiation-induced disorder in the crystal lattice of the film. The gamma radiation can generate a large number of free radicals and ion pairs in the film, which may cause bond breakage and the formation of defects in the crystal lattice. These defects can act as scattering centers, which can reduce the intensity of the diffraction peak.31
The texture coefficient (TC) of plane which is the texture of a certain plane, whose deviation from the standard sample involves the preferred growth was estimated from Eq. 1. The calculated values of the TCs of β-H2Pc film as a function of γ-irradiation doses of the diffraction peak (001) are displayed in Fig. 4b. It is clearly seen that the TC value for (001) plane decreases with increasing the irradiation doses. The lower values of texture coefficient reveals that the films have poor crystallinity.32 Moreover, the gamma radiation can also induce grain boundary formation, which can result in a decrease in the average grain size of the crystallites. The decrease in the grain size can further reduce the intensity of the diffraction peak and increase the hump of amorphism.
In summary, the XRD patterns of both β-H2Pc powder and the film showed gamma irradiation-induced structural changes in the samples. These changes may result from the radiation-induced disorder in the crystal lattice of the samples, the formation of defects, and the reduction in the average grain size. These findings are consistent with the results reported in previous studies on the effect of gamma irradiation on other organic materials.
Figure 2 illustrates the impact of γ-irradiation with various doses on the microstructural parameters of β-H2Pc films. The results show that the mean crystallite size of the films increased with the increasing dose of gamma radiation. In contrast, the microstrain, the average dislocation density, and the number of crystallites per unit surface area decreased as the γ-irradiation doses increased. This behavior can be attributed to the merging of defects during irradiation, leading to the formation of larger grains. The reduction in the microstrain and the average dislocation density suggests a decrease in the lattice defects, which could be due to the enhancement and increment in the crystallinity of the films. Therefore, γ-irradiation of β-H2Pc films resulted in significant changes in their microstructure.
Scanning electron microscopy (SEM) was used to study the surface morphology of pristine and γ-irradiated β-H2Pc at different doses, as shown in Fig. 3. The former displays a two-dimensional surface morphology image, while the latter shows the histogram of the average particle size distribution over the entire surface of β-H2Pc irradiated with 60 kGy (as a representative example). The data indicates that the average particle size increases with increasing γ-irradiation doses. This behavior could be attributed to the coalescence of smaller grains to form larger ones.
The mean grain size was calculated and plotted as shown in Fig. 4a to confirm the effect of γ-irradiation doses on β-H2Pc. The graph clearly shows an increase in the grain size as the doses increase, except for a significant drop at 100 kGy. This could be attributed to the saturation of grain growth due to the limited number of β-H2Pc molecules available for crystal growth. Additionally, at high doses, the radiation-induced defects can promote amorphization, which leads to a decrease in grain size. This is consistent with previous studies that have reported a decrease in grain size at high radiation doses due to the increased amorphization caused by the collision cascades of high-energy particles.
XRD and SEM analyses showed consistent results, with both techniques revealing an increase in grain size and a decrease in microstrain and lattice defects with increasing doses of gamma radiation. SEM images also confirmed an increase in the average particle size of the β-H2Pc films with increasing radiation doses.
These findings suggest that gamma radiation can be used to tailor the microstructure and surface morphology of β-H2Pc films for potential applications in various fields such as optoelectronics, photovoltaics, sensors, and catalysis. For example, changes in the crystallinity and grain size of the β-H2Pc films can affect their charge transport properties, making them suitable for use in electronic devices such as organic field-effect transistors. Similarly, modifications in the surface morphology can enhance the sensitivity of β-H2Pc films to chemical or biological species, making them useful for sensing applications. The use of gamma radiation to tailor the microstructure and surface morphology of β-H2Pc films can therefore open up new avenues for the development of advanced functional materials.
FTIR Analysis
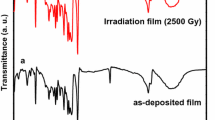
FTIR spectra were utilized in the study to evaluate the chemical stability of β-H2Pc after exposure to γ-irradiation. The spectra for both pristine and irradiated β-H2Pc powders were obtained (Fig. 5). The results indicate that while γ-irradiation has a significant impact on the structure and surface morphology of β-H2Pc, the molecular structure of β-H2Pc remained stable after exposure to γ-irradiation except for the disappearance of the hydroxyl (OH) functional group peak. The presence of the hydroxyl group O-H in the pristine sample may indicate water absorption from the surrounding atmosphere. However, γ-irradiation was observed to have a positive impact on the material by removing the hydroxyl group without affecting the main functional groups of β-H2Pc. Furthermore, the FTIR spectra of the pristine and irradiated powders were quite similar with insignificant differences in band intensities, indicating the molecular stability of β-H2Pc. These findings suggest that β-H2Pc has good radiation resistance and can be utilized in various applications, particularly in medical applications such as cancer treatment.
Photoluminescence Analysis
To evaluate the suitability of β-H2Pc for optoelectronic applications, it is important to study its photoluminescence (PL) characteristics. Figure 6a displays the emission spectrum of both pristine and irradiated β-H2Pc at room temperature (300 K). The emission spectrum was generated using a 300 nm excitation source. It exhibits a strong and broad band of emission in the visible region, corresponding to orange light. The peak of the emission was observed at around 602.02 nm. This high broadband is the result of the direct electronic transition from the first excited state to the ground state accompanied by phonon energy within the inter-band. This phenomenon is responsible for the observed band in the PL spectrum.
Moreover, Fig. 6b reveals the proposed energy level diagram of β-H2Pc. This diagram depicts the energy gap, the electron excitation from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) and the orange emission peak correspond to the electron transitions from the LUMO levels to levels higher than the top of the HOMO levels.
The intensity and broadening of the PL spectra are dependent on the γ-irradiation doses, but all peaks of emission are nearly centered at the same wavelength. As the γ-irradiation doses increase, the intensity of the emission band decreases, indicating the generation of defects in the substance. This is because γ-radiation can create defects in the crystal lattice, leading to a decrease in the PL intensity. This observation is consistent with the results reported in previous studies.33
Linear Optical Characterizations
Absorption Spectrum Analysis
Figure 7a shows the absorption spectra of pristine and irradiated β-H2Pc films in the 200-800 nm wavelength range. The electronic absorption spectra display three main bands corresponding to the maximum absorption at 620 nm, 330 nm, and 212 nm. The Q-band in the wavelength range 540 nm–760 nm corresponds to the π–π* transition from HOMO (Highest occupied molecular orbital) to the LUMO (Lowest unoccupied molecular orbital). The Soret-band (B-band) in the UV region in the 260-380 nm range arises from the deeper π–π* transitions which give the absorption edge. The Q- and B-bands exhibit the characteristic splitting (Davydov splitting) in all thin films, as observed in the literature.34 The third band, the C-band, is in the 190 nm–230 nm wavelength range. An increase in γ-irradiation doses decreases the absorption band intensity, implying that γ-radiation can induce defects in the bulk or thin film. This result is consistent with previous studies that have shown that γ-radiation can cause defects in materials, such as changes in the crystalline structure, vacancies, interstitials, or other types of imperfections. Therefore, careful consideration of γ-irradiation doses is necessary to maintain the quality of materials for their intended application.
Except for the 100 kGy doses, none of the peaks in the absorption spectra of the gamma-irradiated β-H2Pc films showed any significant shift towards shorter or longer wavelengths. Such shifts can indicate changes in the conjugation system or the electronic density of the material, or the formation of new products or intermediates. However, for the 100 kGy doses, all peaks were observed to shift towards higher wavelengths. This shift can be desirable in certain applications, such as the design of more efficient photovoltaic devices, where high-energy absorption is important for converting incident light into electrical energy.
By shifting the absorption peaks of phthalocyanines towards higher energy, a larger portion of the incident light with higher energy (shorter wavelength) can be absorbed, resulting in more efficient energy conversion. This can be achieved through a decrease in the length of the conjugated system or an increase in the electronic density of the molecule, which can be induced by gamma irradiation. Additionally, by tuning the absorption spectra of phthalocyanines, it is possible to match the solar spectrum and maximize the energy conversion efficiency of the photovoltaic device. This is because the solar spectrum varies with the time of day, season, and location, and a photovoltaic device that is optimized for one specific spectral range may not be as effective under different conditions.
Therefore, the ability to induce a shift towards higher energy absorption through gamma irradiation can be beneficial in designing more efficient photovoltaic devices. However, it is important to carefully consider the effects of gamma irradiation on other properties of phthalocyanines such as stability, toxicity, and cost-effectiveness.
Transmission and Reflection Spectra Analysis
The reflectance R and transmittance T spectral behavior of pristine and γ-irradiated β-H2Pc films as a function of wavelength 200 nm-2500 nm are depicted in Fig. 7b. The spectrum can be divided into two regions: the transparent region and the absorbing zone. The transparent region is seen at longer wavelengths (λ > 900 nm), where the films become transparent and no light is absorbed. The summation of R and T is approximately equal to unity in this region. The absorbing zone is observed at wavelengths less than 900 nm, where the films are good absorbers for light waves, and the inequality (T + R < 1) holds. The reflectance R and transmittance T spectral distribution was observed to be affected by γ-irradiation.
Increasing the γ-irradiation doses leads to a slight decrease in transmittance behavior but an increase in reflectance behavior within the non-absorption region. In the absorption region, the intensity of transmission peaks was reduced, while the intensity of reflection peaks increased due to γ-irradiation. Interestingly, no more reflection or transmission peaks are observed in the irradiated films spectrum, which implies that irradiation has not brought any change in the reflectance and transmittance features of β-H2Pc films. This can be attributed to changes in β-H2Pc surface morphology and composition, which can affect the way β-H2Pc interacts with light. Consequently, this leads to alterations in the optical properties of the films within the absorbing region.
Absorption Coefficient and Optical Band Gap Calculation
In Fig. 8a, the dependence of the absorption coefficient on the photon energy for pristine and γ-irradiated β-H2Pc films is depicted. The results suggest that the spectral distribution band intensity of the absorption coefficient decreases as the γ-irradiation doses increase. Notably, the β-H2Pc thin films exhibit a high absorption coefficient value greater than 105 cm-1. A high absorption coefficient means that the material can efficiently absorb a large amount of light energy, which can be useful in applications such as solar cells, photodetectors, and optical filters. This feature makes the β-H2Pc thin films promising candidates for optoelectronic devices, particularly for solar cells, where high absorption is required for efficient energy conversion.
Furthermore, Eq. 8 allows us to estimate the absorption index k for both pristine and γ-irradiated β-H2Pc films based on the absorption coefficient. Also, the absorption index was observed to be decreased as the γ-irradiation doses increase. The spectral distribution of the absorption index for these films is illustrated in Fig. 8b. The results demonstrate that the value of k is higher at shorter wavelengths, which is consistent with previous studies. At longer wavelengths, the value of k approaches zero, but then slightly increases. This slight increase may be attributed to free carrier absorption, a phenomenon that occurs when the carriers in a material absorb photons due to their interaction with the electric field of light.
The higher value of k at shorter wavelengths could be due to the stronger interaction of photons with the material at higher energies. As the wavelength increases, the energy of the photons decreases, resulting in weaker interaction and lower absorption. The increase in k at longer wavelengths could be attributed to the presence of a tail in the absorption spectrum, which can contribute to the absorption coefficient even at longer wavelengths where the absorption is normally low. This phenomenon is often observed in materials with complex electronic structures, such as phthalocyanines. Overall, the behavior of the absorption index suggests that β-H2Pc thin films could be suitable for optoelectronic applications, particularly those that require high absorption at shorter wavelengths.35
On the other side, the reason for the decrease in spectral distribution band intensity of the absorption coefficient and the absorption index as the γ-irradiation doses increase could be due to various factors. One possible reason is that gamma irradiation can induce structural changes in the material, such as breaking chemical bonds and altering the conjugation system, which can affect the electronic structure and density of the material. This, in turn, can lead to a decrease in the absorption coefficient and intensity of the spectral distribution band. Additionally, gamma irradiation can cause oxidation or reduction of the material, leading to changes in the electronic structure and bandgap. It can also cause a reduction in crystal size and induce the formation of defects, impurities, or new phases, which can result in changes to the material’s optical and structural properties. Therefore, there could be multiple cohesive factors that contribute to the decrease in spectral distribution band intensity of the absorption coefficient of β-H2Pc under gamma irradiation.
For further investigation, the determination of energy gap values for both pristine and γ-irradiated β-H2Pc films was calculated using Eq. 11. The exponent s value, which can be used to specify the electronic transition type, was found to be 1/2 for the allowed direct transition and 2 for the indirect allowed transition. The energy band gap (Eg) can be obtained by plotting the relationship between \({(\alpha h\upsilon ) }^{s}\) and hυ. Fig. 9a displays the plot of \({(\alpha h\upsilon ) }^{1/2}\) against hυ for both pristine and γ-irradiated β-H2Pc films. The figure demonstrates the best fit between \({(\alpha h\upsilon ) }^{1/2}\) and hυ, confirming the presence of allowed indirect transition for all films. The energy gap values were obtained from the intercepts with the abscissa (at \({(\alpha h\upsilon ) }^{1/2}=0\)) as shown in Fig. 9a The values of onset energy gap (Eg1) and fundamental energy gap (Eg2) for both pristine and γ-irradiated β-H2Pc films were estimated and tabulated in Table I, along with those of other phthalocyanines. The optical energy gap values as a function of γ-irradiation doses are illustrated in Fig. 9b It is observed that the optical band gap slightly decreases as the γ-irradiation doses increase. This decrease in the optical band gap could be attributed to the changes in the electronic structure or the formation of new energy levels induced by the γ-irradiation.36
Optical Dispersion Parameters Analysis
The determination of refractive index (n) is a significant parameter in the study of optoelectronic devices, which can be estimated using the Eq. 10. The spectral behavior of the refractive index against the wavelength for both pristine and γ-irradiated β-H2Pc films is presented in Fig. 10. The dispersion curves of the refractive index for both regions of λ < 1500 nm and λ > 1500 nm can be analyzed in terms of anomalous and normal behavior, respectively. The anomalous behavior with several peaks in the shorter wavelength spectral region can be anatomized using the multi-oscillator model.37 While the normal behavior with linear refractive index in the longer wavelength spectral region can be interpreted using a single oscillator model.
It is evident from Fig. 10 that there is a slight increase in refractive index values as the γ-radiation doses increase. This trend is observed in all the peaks' intensity in the shorter wavelength spectral region. The increase in refractive index values can be attributed to an increase in mass density and an accumulation of β-H2Pc molecules.17 The remarkable change in the refractive index values with increasing irradiation may be due to the defects generated in the film by gamma radiation and a change in the optical density. Furthermore, it is worth noting that the refractive index pattern for the β-H2Pc in the current study is consistent with the measured reflectance.
Expression (12) can be used to represent the single oscillator model according to Wemple and DiDomonico. Fig. 11a shows the plot of (n2 − 1)− 1 against (hν)2 for both pristine and γ-irradiated β-H2Pc films. To obtain the oscillator energy and dispersion energy values for the films, the intercept and slope of the curve were determined using the single oscillator model expressed by Wemple and DiDomonico. These values were then compared to those of other Pcs compounds and summarized in Table I for further analysis. Fig. 11b displays the change in Ed and Eo values with gamma irradiation doses for β-H2Pc thin films. Notably, both Ed and Eo values decreased as the gamma radiation exposure increased for β-H2Pc thin films. The decrease in oscillator and dispersion energy values observed in the β-H2Pc thin films can be explained by the formation of defects or impurities in the material due to the ionizing effect of gamma radiation. These defects and impurities can alter the electronic structure of the material, leading to a weakening of the intermolecular interactions between the molecules in the material.38
Furthermore, the infinite frequency dielectric constant ε∞ can be calculated from the intercept at (hν)2 = 0 using the estimated values of the dispersion parameters. The values of ε∞ for both pristine and γ-irradiated β-H2Pc films are computed and presented in Table I alongside the previously obtained results for other Pcs compounds.
Equation 13 gives the variation between the lattice dielectric constant εL and the square of the wavelength in the transparent region. The spectral dependence of n2 on λ2 is shown in Fig. 12a. The values of εL were estimated by extrapolating the plot to λ2 = 0. Also, the value of N/m* was determined from the slope of the resulting straight line. These estimated values are listed in Table I and compared with those of other Pcs compounds. Fig. 12b presents the changes in ε∞ and εL with gamma irradiation doses for β-H2Pc thin films, where it is observed that both values increase with increasing doses. Fig. 12c shows the dependence of N/m* on gamma irradiation doses for β-H2Pc thin films. It is seen that the N/m* ratio increases with increasing doses. The main reason for the discrepancy between the infinite frequency dielectric constant and lattice dielectric constant values is attributed to the generation of free charges inside the material during the polarization process.39
Dielectric Characterizations
The complex dielectric function is a crucial parameter that characterizes the optical response of a material to photon energy. It provides information on how light propagates, reflects, and gets absorbed within the medium. The real and imaginary parts of the dielectric constant can be obtained from the Eqs. 14, 15 which are derived from n and k.
In the case of pristine and γ-irradiated β-H2Pc films, the variation of the real (ε1) and imaginary (ε2) parts of the dielectric constant with photon energy is shown in Fig. 13a and b, respectively. The presence of peaks in the dielectric spectra indicates the type of material and its electronic structure through the interaction between photons and electrons. The changes in the dielectric constant values as a function of photon energy signify that interactions between electrons and photons occur within the film in this range of energy. These interactions give rise to peaks in the dielectric spectra.
The real part of the dielectric constant spectrum describes the material’s response to an electric field at different photon energies. The given observation suggests that as the gamma irradiation dose increases, the material's response to the electric field decreases in the energy range between 2 eV to 4 eV. This could be due to the formation of defects in the material that hinders its ability to respond to the electric field at these energies. This could be due to the formation of defects in the material that hinders its ability to respond to the electric field at these energies. However, at energies higher than 4 eV, the spectrum intensity increases. This could be due to the formation of additional electronic states in the films that can respond to the electric field at these higher energies.
On the other hand, the imaginary part of the dielectric constant spectrum describes the material's absorption of light at different energies. The observation that the intensity of the peaks in the imaginary part of the dielectric constant decreases with increasing gamma irradiation doses suggests that the material's ability to absorb light at specific energies is reduced. This could also be due to the formation of defects that impede the absorption of light at these energies.
Furthermore, the dissipation factor of pristine and γ-irradiated β-H2Pc films was estimated using Eq. 16 and plotted against the incident photon energy in Fig. 14. It was revealed that increasing gamma radiation doses decrease the dissipation factor band's intensity. The reduction in the dissipation factor with increasing gamma dose can be attributed to the damage that gamma radiation causes to the molecular structure of the dielectric material. This damage results in a diminished ability of the molecules to reorient themselves in response to the applied electric field, leading to less energy being dissipated by the material. These changes in the electronic structure caused by the gamma radiation can affect the polarization behavior of the molecules, resulting in a decrease in the dissipation factor.
The characteristic energy loss of free carriers moving within the films and surface was also studied. The volume energy loss function (VELF) and surface energy loss energy (SELF) for a thin film were calculated using Eqs. 17 and 18. Their dependence on photon energy for both pristine and γ-irradiated β-H2Pc films was depicted in Fig. 13c and d, respectively. The results showed no significant difference between VELF and SELF at lower and higher photon energies, with energy loss being primarily caused by free charge carriers traversing through the bulk material (VELF) rather than the surface (SELF). Interestingly, the intensity of both VELF and SELF bands increased up to 4.5 eV as gamma radiation doses increased, but then decreased for higher photon energies. This suggests that gamma radiation initially induced additional free charge carriers in the material, leading to an increase in the energy loss functions, but higher doses caused damage to the molecular structure, leading to a reduction in energy loss.
Optical Conductivity Analysis
The study of complex optical conductivity is important in understanding the behavior of a material in response to light. The relationship between the complex dielectric constant and the complex optical conductivity can be described by Eqs. 19 and 20. In this section, the real and imaginary parts of the optical conductivity for pristine and γ-irradiated β-H2Pc films were analyzed and presented in Fig. 13e and f, respectively. These figures provide insight into the material's electronic structure and the nature of the transitions responsible for the absorption. The changes in the optical conductivity of β-H2Pc films with increasing gamma radiation doses can be attributed to the fact that gamma radiation can cause damage to the molecular structure of the material. This damage can lead to changes in the electronic structure of the material, which in turn affects its optical properties.
In the case of β-H2Pc films, the real part of the optical conductivity is related to the ability of the material to conduct electricity in response to an applied electric field. As the gamma radiation dose increases, the damage to the molecular structure of the material reduces its ability to conduct electricity, resulting in a decrease in the real part of the optical conductivity. On the other hand, the imaginary part of the optical conductivity is related to the ability of the material to absorb light. As the gamma radiation dose increases, the damage to the molecular structure of the material can lead to an increase in the number of electronic transitions that result in the absorption of light, increasing the imaginary part of the optical conductivity.
Moreover, the analysis revealed that both the pristine and γ-irradiated β-H2Pc films exhibit higher values of the imaginary part of optical conductivity as compared to the real part. Such behavior is generally considered unfavorable for optoelectronic applications, where a higher real part of optical conductivity is desirable for efficient light absorption and conversion. A higher imaginary part signifies higher absorption losses, leading to a decrease in the efficiency of the device. Therefore, the observed behavior of β-H2Pc films indicates the need for further optimization to improve their performance in optoelectronic applications.
To sum up, our analysis of the optical conductivity of both pristine and γ-irradiated β-H2Pc films revealed that the real part of the optical conductivity decreases while the imaginary part increases as the gamma radiation doses increase. This behavior is similar to that of some other materials used in optoelectronic applications, such as ZnO and CdSe, while being opposite to that of some metal pc materials, such as CuPc and FePc. However, both the pristine and irradiated films exhibit a higher imaginary part of the optical conductivity than the real part, which is not a favorable behavior for optoelectronic devices. Our findings are consistent with the existing literature on the optical properties of β-H2Pc films and provide valuable insights into the behavior of these materials under gamma radiation. Future research can focus on optimizing the synthesis and fabrication processes to improve the optical properties of β-H2Pc films for practical optoelectronic applications.
Nonlinear Optical Analysis
Organic materials with high nonlinear optical susceptibility are highly desirable in the development of advanced optoelectronic devices. The nonlinear optical refractive index provides valuable insight into the interaction of light with these materials. The ability of a material to generate a nonlinear optical response, particularly at the third harmonic of the incident light, can be quantified by its nonlinear third-order optical susceptibility χ(3) and nonlinear optical refractive index n2. Both parameters can be evaluated using Eqs. 21 and 22. The obtained values for these parameters for both pristine and irradiated β-H2Pc films are presented in Table I, along with corresponding phthalocyanine compounds. Observations reveal that β-H2Pc films exhibit significantly higher χ(3) and n2 values when compared with values reported for other materials in the literature. This indicates that β-H2Pc films have great potential for various nonlinear optical applications, making them a promising candidate for optoelectronic devices such as solar cells and Organic Light Emitting Diodes (OLEDs). The higher χ(3) and n2 values of β-H2Pc films may be attributed to the presence of defect centers induced by gamma irradiation, which enhances local polarizabilities and leads to an increase in nonlinear optical response.
The spectral distribution of χ(3) and n2 versus photon energy for both pristine and γ-irradiated β-H2Pc films is shown in Fig. 15a and b, which displays similar behavior with three peaks and an irradiation dependency. The presence of peaks in the nonlinear optical susceptibility at specific photon energies indicates the existence of distinct physical mechanisms contributing to the overall nonlinear response of the material. In the case of β-H2Pc films, the three peaks at 0.75 eV, 1.60 eV, and 2 eV are attributed to different physical mechanisms. The peak at 0.75 eV is associated with the one-photon resonant electronic transitions between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). The peak at 1.60 eV could be attributed to the two-photon resonant electronic transitions. The peak at 2 eV could be explained by the combination of one-photon and two-photon electronic transitions with a significant contribution from the two-photon absorption process. The presence of multiple peaks in the nonlinear optical susceptibility provides important information about the underlying physical mechanisms contributing to the nonlinear response of the material. By understanding the physical mechanisms involved, researchers can tailor the material's properties to optimize its performance for specific applications.
Additionally, the results illustrated in Fig. 15c reveal that the values of χ(3) and n2 increase proportionally with the gamma irradiation dose. The enhancement in these values can be attributed to the formation of defect centers within the material due to external gamma irradiation. These defect centers can significantly increase the local polarizabilities of the material, which results in a larger nonlinear optical response. As a result, β-H2Pc films are highly suitable for various nonlinear optical applications. The significance of these effects can be observed in various fields such as telecommunications, materials science, and bio-photonics.
Conclusion
According to the obtained results, γ-irradiation can be a useful tool for adjusting the characteristics of nanocrystalline β-H2Pc thin films for particular applications at appropriate dosages. The γ-irradiation irradiation-induced disorder in the film's crystal lattice caused the crystal structure to be compromised, which explains the reductions of (001) peak with increasing γ-irradiation exposure. The ability of gamma radiation to induce a shift towards higher energy absorption could be beneficial in designing more efficient photovoltaic devices. However, it is important to consider the effects of gamma irradiation on other properties of β-H2Pc such as stability, toxicity, and cost-effectiveness. As the dose of γ-irradiation increases, defects in nanocrystalline β-H2Pc thin films cause an increase in the dispersion and oscillating parameters. The presented study offers helpful information about the potential applications of β-H2Pc thin films in optoelectronic components, particularly solar cells, where high absorption is necessary for effective energy conversion. In our future work, we may use the outcomes of this study to design novel factors and procedures that have a significant impact on solar cell efficiency.
References
Y.J. Yang, S.X. Li, D.L. Chen, and Z.W. Long, Geometric structure, electronic, and spectral properties of metal-free phthalocyanine under the external electric fields. ACS Omega 7, 41266 (2022).
A.A. El-Saady, N. Roushdy, A.A.M. Farag, M.M. El-Nahass, and D.M. Abdel Basset, Exploring the molecular spectroscopic and electronic characterization of nanocrystalline metal-free phthalocyanine: a DFT investigation. Opt. Quantum Electron. 55, 662 (2023).
S.A. Al-Ghamdi, T.A. Hamdalla, E.F.M. El-Zaidia, A.O.M. Alzahrani, N. Alghamdi, S. Khasim, I.S. Yahia, and A.A.A. Darwish, Structural, electronic, and optoelectronic characteristics of GaClPc/n-Si heterojunction for photodiode device. Mater. Sci. Semicond. Process. 147, 106704 (2022).
G. Gümüşgöz Çelik, G. Tunç, F. Lafzi, N. Saracoglu, B. Seçkin Arslan, M. Nebioğlu, İ Şişman, and A. Gül Gürek, Influence of spacer and donor groups as tetraphenylethylene or triphenylamine in asymmetric zinc phthalocyanine dyes for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 444, 114962 (2023).
A. Stachowiak, K. Kędzierski, B. Barszcz, K. Kotwica, and D. Wróbel, Determination of phthalocyanines energy gaps based on spectroscopic and electrochemical studies and DFT calculations. J. Mol. Liq. 341, 116800 (2021).
X. Rozhkova, A. Aimukhanov, A. Zeinidenov, V. Paygin, D. Valiev, J. Bisquert, A. Guerrero, A. Alexeev, and B. Ilyassov, Nanocomposition of PEDOT:PSS with metal phthalocyanines as promising hole transport layers for organic photovoltaics. Synth. Met. 295, 117347 (2023).
G. Guney and S. Gorduk, Photophysical and Photochemical studies on non-peripherally and peripherally Ga(III) chloro phthalocyanines in different solvents. J. Organomet. Chem. 999, 122813 (2023).
E. Yabaş, E. Biçer, and R. Katırcı, Experimental and in silico studies on optical properties of new thiadiazole tetrasubstituted metal-free and zinc phthalocyanine compounds. Opt. Mater. 122, 111808 (2021).
M.M. El-Nahass, K.F. Abd-El-Rahman, H.M. Zeyada, and A.A.A. Darwish, Influence of γ-irradiation on the optical parameters of 4-tricyanovinyl-N, N-Diethylaniline thin films. Opt. Commun. 285, 2864 (2012).
A. Morana, E. Marin, L. Lablonde, T. Blanchet, T. Robin, G. Cheymol, G. Laffont, A. Boukenter, Y. Ouerdane, and S. Girard, Radiation Effects on fiber bragg gratings: vulnerability and hardening studies. Sensors 22, 8175 (2022).
S.J. Yaghmour, Influence of γ-irradiation on optical properties of manganese phthalocyanine thin films. J. Alloys Compd. 486, 284 (2009).
A.A.A. Darwish, S.A.M. Issa, and M.M. El-Nahass, Effect of gamma irradiation on structural, electrical and optical properties of nanostructure thin films of nickel phthalocyanine. Synth. Met. 215, 200 (2016).
M.M. El-Nahass, A.A. Atta, E.A.A. El-Shazly, A.S. Faidah, and A.A. Hendi, Influence of γ-irradiation on the optical properties of nanocrystalline tin phthalocyanine thin films. Mater. Chem. Phys. 117, 390 (2009).
M.M. El-Nahass, A.A.M. Farag, and A.A. Atta, Influence of heat treatment and gamma-rays irradiation on the structural and optical characterizations of nano-crystalline cobalt phthalocyanine thin films. Synth. Met. 159, 589 (2009).
M.M. El-Nahass, B.A. Khalifa, and I.M. Soliman, Gamma radiation-induced changes on the structural and optical properties of aluminum phthalocyanine chloride thin films. Opt. Mater. 46, 115 (2015).
H.M. El- Mallah, M. Abd- El Salam, D.G. El- Damhogi, and E. Elesh, Structural characterization and optical parameter of silicon phthalocyanine dichloride thin films dependence with gamma ray radiation. Radiat. Phys. Chem. 176, 109012 (2020).
D.G. El- Damhogi, E. Elesh, A.H. Ibrahim, S. Mosaad, M.M. Makhlouf, and Z. Mohamed, The impact of radiation on the morphological, structural properties, linear and nonlinear optical parameters of gallium phthalocyanine chloride thin films for optoelectronic devices. Radiat. Phys. Chem. 195, 110060 (2022).
H.G. Mohammed, T. Mohammed Badri Albarody, H. Kareem Mohsin Al-Jothery, M. Mustapha, and N. Sultan, A study of crystalline – texture and anisotropic properties of hexagonal BaFe12O19 sintered by in-situ magnetic-anisotropy spark plasma sintering (MASPS). J. Magn. Magn. Mater. 553, 169268 (2022).
S.J. Alsufyani, A.N. Alharbi, A.A. Atta, T.A. Altalhi, M.S. Refat, A.A. Alkathiri, A. Ashour, and A.M. Hassanien, A spectroscopic study and the effect of gamma rays on the stability and efficiency of boron subphthalocyanine dye for solar energy applications. Radiat. Phys. Chem. 208, 110929 (2023).
E.F.M. El-Zaidia, R. Bousbih, A.A.A. Darwish, S.I. Qashou, Z.M. Mohammedsaleh, Z. Bassfar, I.S. Yahia, and F.S. Abu-Samaha, Effect of film thickness on structural, electrical and optical properties of amorphous boron subphthalocyanine chloride thin film. Opt. Mater. 138, 113691 (2023).
S. Aldawood, O.M. AlTalib, M.S. AlGarawi, T.S. Alkhuraiji, Y. Alashban, N. Shubayr, A.T. Abdul Rahman, K. Saeed, and S.M. Ali, Gamma ray effects on the properties of PbI2 thin films. Radiat. Phys. Chem. 193, 110003 (2022).
A.M.A. Shamekh, N.M. Shaalan, T.A. Hanafy, and M. Rashad, Linear/nonlinear optical properties of functional inorganic MgO nano-filler in PVA transparent polymer for flexible optoelectronic devices. Phys. B Condens. Matter 651, 414617 (2023).
A.M. Hassanien, A.A.A. Darwish, A.M. Kamal, M. Al-Gawati, and T.A. Hamdalla, Annealing Effect on the morphology, linear and non-linear optical properties of squaraine derivative thin films for optoelectronics applications. Opt. Mater. 142, 114033 (2023).
S. Alfadhli, A.A.A. Darwish, S. Soliman, E.F.M. El-Zaidia, I.S. Yahia, F. Laariedh, A. Alatawi, A. Bahamran, N.M. Alatawi, and T.A. Hamdalla, Structural characterizations and photoelectric performance of non-crystalline boron subphthalocyanine chloride films/FTO for photodiode applications. J. Non. Cryst. Solids 601, 122044 (2023).
G.B. Sakr, I.S. Yahia, M. Fadel, S.S. Fouad, and N. Romević, Optical spectroscopy, optical conductivity, dielectric properties and new methods for determining the gap states of CuSe thin films. J. Alloys Compd. 507, 557 (2010).
A.A.A. Darwish, T.A. Hamdalla, E.F.M. El-Zaidia, T.A. Hanafy, S.A.M. Issa, and I.S. Yahia, Thin films of nanostructured Gallium (III) chloride phthalocyanine deposited on FTO: structural characterization, optical properties, and laser optical limiting. Phys. B Condens. Matter 593, 412321 (2020).
A.A.M. Farag, M. Abdel Rafea, N. Roushdy, O. El-Shazly, and E.F. El-Wahidy, Influence of Cd-content on structural and optical dispersion characteristics of nanocrystalline Zn1-XCdxS (0 ≤ x ≤ 0.9) films. J. Alloys Compd. 621, 434 (2015).
S.A.A. Alghamdi, A.A.A.A.A. Darwish, I.S.S. Yahia, and E.F.M.F.M. El-Zaidia, Structural characterization and optical properties of nanostructured Indium (III) phthalocyanine chloride/FTO thin films for photoelectric applications. Optik (Stuttg). 239, 166780 (2021).
E.R. Sharaf, I.S. Yahia, M.I. Mohammed, H.Y. Zahran, and E.R. Shaaban, High refractive index and third-order nonlinear optical susceptibility of nanostructured ZnSe/FTO thin films: towards smart multifunctional optoelectronic materials. Phys. B Condens. Matter 602, 412595 (2021).
O.I. Sallam, M.I.A. Abdel Maksoud, S.M. Kassem, A.S. Awed, and N.A. Elalaily, Enhanced linear and nonlinear optical properties of erbium/ytterbium lead phosphate glass by gamma irradiation for optoelectronics applications. Appl. Phys. A 128, 819 (2022).
A. Badawi, S.J. Alsufyani, S.S. Alharthi, M.G. Althobaiti, A.A. Alkathiri, M. Almurayshid, and A.N. Alharbi, Impact of gamma irradiation on the structural, linear and nonlinear optical properties of lead oxide incorporated PVA/graphene blend for shielding applications. Opt. Mater. 127, 112244 (2022).
S. Thanikaikarasan, T. Mahalingam, M. Raja, T. Kim, and Y.D. Kim, Characterization of electroplated FeSe thin films. J. Mater. Sci. Mater. Electron. 20, 727 (2009).
M.A. Ibrahim, A.A.M. Farag, N. Roushdy, and N.M. El-Gohary, Synthesis, optical and photoelectrical characterizations of the novel 10-chloro-6H,8H-dichromeno[2,3-b:3′,4′-e]pyridine-6,8-dione (CDPD) and its photodiode application. Opt. Mater. 51, 70 (2016).
A.A.M. Farag, A.M. Mansour, A.H. Ammar, M.A. Rafea, and A.M. Farid, Electrical conductivity, dielectric properties and optical absorption of organic based nanocrystalline sodium copper chlorophyllin for photodiode application. J. Alloys Compd. 513, 404 (2012).
A.A.M. Farag, N. Roushdy, N.M. El-Gohary, S.A. Halim, and M.A. Ibrahim, Synthesis, DFT studies and photovoltaic characteristics of 2-Amino-N-Cyclohexyl-5-Oxo-5H-Chromeno[2,3-b]Pyridine-3-carboxamide (ACCP). Appl. Surf. Sci. 467–468, 1226 (2019).
F.A. Mir, A. Gani, and K. Asokan, Gamma irradiation studies of composite thin films of poly vinyl alcohol and coumarin. RSC Adv. 6, 1554 (2016).
E.F.M. El-Zaidia, Studies structure, surface morphology, linear and nonlinear optical properties of nanocrystalline thin films of Manganese (III) phthalocyanine chloride for photodetectors application. Sens. Actuators A Phys. 330, 112828 (2021).
F.A. Najar, F.A. Mir, G.B. Vakil, S.A. Dar, and B. Ghayas, Effect of γ-radiations on the optoelectrical parameters of coumarin-poly vinyl alcohol composite thin films. Radiat. Phys. Chem. 193, 109973 (2022).
M.M. El-Nahass and A.A.M. Farag, Structural, optical and dispersion characteristics of nanocrystalline GaN films prepared by MOVPE. Opt. Laser Technol. 44, 497 (2012).
M.M. El-Nahass, A.M. Farag, K.F. Abd El-Rahman, and A.A.A. Darwish, Dispersion studies and electronic transitions in nickel phthalocyanine thin films. Opt. Laser Technol. 37, 513 (2005).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
AAE-S: involved in preparing the samples, optical spectroscopic measurement, formal analysis of X-ray, SEM, FTIR, photoluminescence and optical properties measurements, writing-original draft, and final revision of the manuscript plagiarism. MME-N: took part in preparing the samples and final revision of the manuscript. Ahmed Ashour: irradiated Samples and x-ray measurement. NR: involved in FTIR, SEM and photoluminescence measurements. AAMF and DMAB: took part in final revision of all the figures and the manuscript writing and manuscript plagiarism.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Saady, A.A., Roushdy, N., Farag, A.A.M. et al. Influence of Gamma-irradiation on the Structural, Morphological, and Optical Properties of β-H2Pc Nanocrystalline Films: Implications for Optoelectronic Applications. J. Electron. Mater. 52, 8001–8018 (2023). https://doi.org/10.1007/s11664-023-10703-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-023-10703-4