Abstract
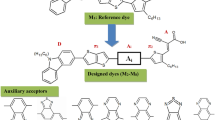
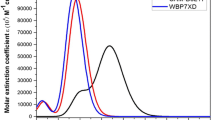
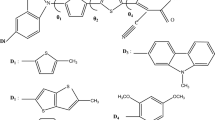
Significant progress has been made in developing organic compounds by modifying carbazole (Cz) based donor–spacer–acceptor (D–π–A) type dye molecules. The Cz-1–Cz-3 dyes were theoretically designed and experimentally synthesized successfully. Herein, we report the synthesis, photophysical properties and electrochemical characterization (quasi-reversible oxidation processes) of three Cz-based compounds. The calculated results agree well with the available experimental data of absorption spectra, HOMO–LUMO energy levels and band gaps using the time-dependent density functional theory. Furthermore, the results from natural bond orbital calculations were analyzed with the computational electronic structure and charge transfer (conjugative interaction) in molecular systems. The result shows fluorescence time-correlated single-photon counting between 4.32 ns, 4.25 ns, and 13.2 ns, and green to blue (λPL = 431–881 nm) emission for complexes Cz-1–Cz-3 in DMF solution. The Cz-3 compound showed excellent photovoltaic properties, with power conversion efficiency of 5.68%. These results clearly reveal that modification of the electron-withdrawing capability in D–π–A conjugated metal-free organic dyes is an effective way to improve the optical and electrical properties of organic photovoltaic (PV) devices.
Graphical Abstract













Similar content being viewed by others
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also form part of an ongoing study.
References
B. O’Regan and M. Grätzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353(6346), 737–740 (1991).
X. Zhang, F. Gou, J. Shi, H. Gao, C. Xu, Z. Zhu, and H. Jing, Molecular engineering of new phenothiazine-based D-A–π–A dyes for dye-sensitized solar cells. RSC Adv. 6(108), 106380–106386 (2016).
Z.S. Wang, Y. Cui, K. Hara, Y. Dan-oh, C. Kasada, and A. Shinpo, A high-light-harvesting-efficiency coumarin dye for stable dye-sensitized solar cells. Adv. Mater. 19(8), 1138–1141 (2007).
B. Liu, W. Zhu, Q. Zhang, W. Wu, M. Xu, Z. Ning, Y. Xie, and H. Tian, Conveniently synthesized isophorone dyes for high efficiency dye-sensitized solar cells: tuning photovoltaic performance by structural modification of donor group in donor–π–acceptor system. Chem. Commun. 13, 1766–1768 (2009).
C. Zafer, M. Kus, G. Turkmen, H. Dincalp, S. Demic, B. Kuban, Y. Teoman, and S. Icli, New perylene derivative dyes for dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 91(5), 427–431 (2007).
X. Ma, J. Hua, W. Wu, Y. Jin, F. Meng, W. Zhan, and H. Tian, A high-efficiency cyanine dye for dye-sensitized solar cells. Tetrahedron 64(2), 345–350 (2008).
S. Hayashi, M. Tanaka, H. Hayashi, S. Eu, T. Umeyama, Y. Matano, Y. Araki, and H. Imahori, Naphthyl-fused π-elongated porphyrins for dye-sensitized TiO2 cells. J. Phys. Chem. C 112(39), 15576–15585 (2008).
P. Shen, Y. Liu, X. Huang, B. Zhao, N. Xiang, J. Fei, L. Liu, X. Wang, H. Huang, and S.T. Tan, Efficient triphenylamine dyes for solar cells: effects of alkyl-substituents and π-conjugated thiophene unit. Dyes Pigm. 83(2), 187–197 (2009).
F. Sanda, T. Nakai, N. Kobayashi, and T. Masuda, Synthesis of polyacetylenes having pendant carbazole groups and their photo-and electroluminescence properties. Macromolecules 37(8), 2703–2708 (2004).
L. Zhao, P. Wagner, A.B.S. Elliott, M.J. Griffith, T.M. Clarke, K.C. Gordon, S. Moric, and A.J. Mozer, Enhanced performance of dye-sensitized solar cells using carbazole-substituted di-chromophoric porphyrin dyes. J. Mater. Chem. A 2, 16963–16977 (2014).
J. Sivanadanam, P. Ganesan, P. Gao, M.K. Nazeeruddin, A. Emeline, D. Bahnemann, and R. Rajalingam, Impact of strength and size of donors on the optoelectronic properties of D–π–A sensitizers. RSC Adv. 6(44), 37347–37361 (2016).
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, J. Honda, O. Kitao, H. Nakai, T. Vreven Jr., J.A. Montgomery, J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.M. Iyengar, J. Tomasi, M. Cossi, R. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, O. Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski, and D.J. Fox, Gaussian 09 Revision A02 (Wallingford: Gaussian Inc, 2009).
A.D. Becke, Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys. 96(3), 2155–2160 (1992).
A.D. Becke, Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098 (1988).
C. Lee, W. Yang, and R.G. Parr, Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785 (1988).
A.E. Reed and F. Weinhold, Natural localized molecular orbitals. J. Chem. Phys. 83(4), 1736–1740 (1985).
S. Miertuš, E. Scrocco, and J. Tomasi, Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 55(1), 117–129 (1981).
S. Miertus and J. Tomasi, Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem. Phys. 65(2), 239–245 (1982).
M. Cossi, V. Barone, R. Cammi, and J. Tomasi, Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem. Phys. Lett. 255(4–6), 327–335 (1996).
R.I. Dennington, T. Keith, and J. Millam, GaussView, Version 508 (Shawnee: Semichem. Inc, 2009).
R.G. Pearson, Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. 83(22), 8440–8441 (1986).
R.V. Solomon, P. Veerapandian, S.A. Vedha, and P.A. Venuvanalingam, Tuning nonlinear optical and optoelectronic properties of vinyl coupled triazene chromophores: a density functional theory and time-dependent density functional theory investigation. J. Phys. Chem. A 116(18), 4667–4677 (2012).
Y. Xue, Y. Dou, L. An, Y. Zheng, L. Zhang, and Y. Liu, Electronic structure and spectral properties of aurones as visible range fluorescent probes: a DFT/TDDFT study. RSC Adv. 6(9), 7002–7010 (2016).
G. Gece, The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 50(11), 2981–2992 (2008).
R.G. Parr, L.V. Szentpaly, and S. Liu, Electrophilicity index. J. Am. Chem. Soc. 121(9), 1922–1924 (1999).
P.K. Chattaraj, B. Maiti, and U. Sarkar, Philicity: a unified treatment of chemical reactivity and selectivity. J. Phys. Chem. A 107(25), 4973–4975 (2003).
R.G. Parr, R.A. Donnelly, M. Levy, and W.E. Palke, Electronegativity: the density functional viewpoint. J. Chem. Phys. 68(8), 3801–3807 (1978).
R.G. Parr and R.G. Pearson, Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 105(26), 7512–7516 (1983).
R.G. Parr and P.K. Chattaraj, Principle of maximum hardness. J. Am. Chem. Soc. 113(5), 1854–1855 (1991).
T. Iijima, A. Momotake, Y. Shinohara, T. Sato, Y. Nishimura, and T. Arai, Excited-state intramolecular proton transfer of naphthalene-fused 2-(2′-hydroxyaryl) benzazole family. J. Phys. Chem. A 114(4), 1603–1609 (2010).
X. Ren, J. Li, R.J. Holmes, P.I. Djurovich, S.R. Forrest, and M.E. Thompson, Ultrahigh energy gap hosts in deep blue organic electrophosphorescent devices. Chem. Mater. 16(23), 4743–4747 (2004).
C. Fan, Y. Wei, D. Ding, and H. Xu, Linkage engineering in hosts for dramatic efficiency enhancement of blue phosphorescent organic light-emitting diodes. Opt. Express 23(10), 12887–12899 (2015).
T. Daeneke, T.-H. Kwon, A.B. Holmes, N.W. Duffy, U. Bach, and L. Spiccia, High-efficiency dye-sensitized solar cells with ferrocene-based electrolytes. Nat. Chem. 3(3), 211–215 (2011).
S.J. Su, C. Cai, and J. Kido, RGB phosphorescent organic light-emitting diodes by using host materials with heterocyclic cores: effect of nitrogen atom orientations. Chem. Mater. 23(2), 274–284 (2010).
E. Scrocco and J. Thomasi, Electronic molecular structure, reactivity and intermolecular forces: an euristic interpretation by means of electrostatic molecular potentials. J. Adv. Quantum Chem. 11, 115–193 (1978).
S.S. Amiri, S. Makarem, H. Ahmar, and S. Ashenagar, Theoretical studies and spectroscopic characterization of novel 4-methyl-5-((5-phenyl-1,3,4-oxadiazol-2-yl) thio) benzene-1, 2-diol. J. Mol. Struct. 1119, 18–24 (2016).
Acknowledgments
We are greatly thankful to I. Ragavan, Research Scholar, Department of Physics, Periyar University, Salem-11, Tamilnadu, India, for his valuable suggestions and strong encouragement to the work. The authors extend their appreciation to the Research Center for Advanced Materials Science (RCAMS), King Khalid University, Saudi Arabia, for funding this work under Grant Number RCAMS/KKU/020-22.
Author information
Authors and Affiliations
Contributions
KP, IR, AA and CV: Design work, experimental processes, conceptualization; computational investigation; methodology; data curation; formal analysis; writing—original draft. PS and PMA: Supervision; software; review and editing; conceptualization; validation; data curation, formal analysis. MS, VRMR and VB, WKK: English language in the revisions to the manuscript; funding acquisition, writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Periyasamy, K., Sakthivel, P., Ragavan, I. et al. Design, Synthesis, and Optical and Electrochemical Properties of D–π–A Type Organic Dyes with Carbazole-Based Donor Units for Efficient Dye-Sensitized Solar Cells: Experimental and Theoretical Studies. J. Electron. Mater. 52, 2525–2543 (2023). https://doi.org/10.1007/s11664-023-10210-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-023-10210-6