Abstract
Rutile-TiO2 ceramics are very promising electronic ceramic materials which can be applied in varistors because of their good electrical properties. In this work, Dy2O3 was used to improve the electrical properties of a TiO2 varistor doped with 0.60 mol.% Nb2O5 and 0.30 mol.% SiO2. Results showed that the TiO2 varistor doped with 0.30 mol.% Dy2O3 possessed optimal electrical properties with good nonlinear coefficient (\( \alpha = 5.7 \)), very low breakdown voltage (\( E_{\text{B}} = 1.34\;{\text{V}}\,{\text{mm}}^{ - 1} \)) and colossal dielectric constant (\( \varepsilon_{\text{r}} = 8.01 \times 10^{5} \)). The microstructural analysis showed that after the incorporation of Dy2O3 into the TiO2 varistor, the grains tended to be more semiconducting due to the defect carriers derived from the substitution of Dy3+ for Ti4+. Meanwhile, the moderate dopant of Dy2O3 also increased the grain size and narrow grain boundary. Consequently, the breakdown voltage decreased, while the nonlinear coefficient and dielectric constant improved.
Similar content being viewed by others
Introduction
Titanium dioxide ceramic is a kind of electronic ceramic that can attain good nonlinear electrical properties and dielectric constant by special methods, which makes it of great interest for a broad application concerning varistors and capacitors. In the past, the ZnO varistor was most widely used owing to its excellent nonlinear characteristics.1 However, the ZnO varistor cannot be applied in low-voltage electronic circuits because of its high breakdown voltage.2 In recent years, with the miniaturization and integration of electronic circuits, it is necessary to develop multifunctional varistors with low breakdown voltage.3,4,5 As one of the typical electronic ceramics with multifunction, the TiO2 varistor has attracted extensive attention due to its low breakdown voltage and good dielectric properties. However, the application of the TiO2 varistor is limited by its low nonlinear coefficient and relatively high breakdown voltage for electronic circuits.
In theory, the breakdown voltage and nonlinear coefficient of the TiO2 varistor mainly depend on the potential barrier and the grain size of TiO2 ceramics.1,6,7 In order to improve the nonlinear electrical properties of TiO2 ceramics, most researchers concentrated on donor doping (Nb5+,Ta5+) and acceptor doping (Ho3+,Cr3+).8,9 In addition, the influences of sintering temperature and heat treatment atmosphere on the electrical properties of the TiO2 ceramic were also studied.10,11,12 In 1995, Yan and Wu found that doping 0.25 at.% Nb5+ was beneficial to the varistor properties of (Ba-Bi-Nb)-doped TiO2.13 Meanwhile, Luo et al. reported that Ta2O5 could effectively improve the properties of (Ca, Si, Ta)-doped TiO2 varistor.14 These two reports confirmed that donor doping could effectively enhance the varistor properties of TiO2 ceramics, but the high breakdown voltage still limited its application. Sousa et al. investigated the effect of Ta2O5 as well as Cr2O3 on the varistor properties of TiO2 9 and Kang et al. reported the (Ge, GeO2, Ta2O5, BaCO3) co-doped TiO2 varistor with an α value up to 12.1 and a low breakdown voltage of 20.8 V/mm.15 Although both donor doping and acceptor doping were shown helpful to the electrical properties of the TiO2 varistor, the difficulties of coordinating the breakdown voltage and nonlinear coefficient, i.e., how to further reduce the breakdown voltage and enhance the nonlinear coefficient simultaneously, has become a challenge for researchers. In addition, heat treatment atmospheres also had a favorable effect on TiO2 varistor properties. For instance, Zhao et al. implied that after the TiO2 varistor was heat-treated in oxygen atmosphere, the acceptor state density as well as the height and width of the potential barriers increased, the conductivity decreased and the breakdown voltage rose significantly as a consequence of oxygen accumulation at grain boundaries.10
Previous studies on TiO2 ceramics mainly focused on the improvement of nonlinear properties. However, recently, researchers found that the doping of In3+ + Nb5+ (1:1) into TiO2 ceramics (INTO) produced colossal dielectric properties, making the TiO2 ceramics a potential capacitor.16 Subsequently, Thongbai et al. reported the effects of sintering conditions and doping concentrations on Sc3+ + Nb5+ (1:1) co-doped TiO2 ceramics and found that Sc3+ captured free electrons at internal insulating layers while Nb5+ produced free electrons. The work revealed that the interfacial polarization at insulating layers and large activation energy contributed to colossal dielectric properties of ScNTO ceramics.17 Lately, there has been much research about Eu3+ + Nb5+,18 La3+ + Ta5+,19 Er3+ + Ta5+,20 Bi3+ + Eu3+ + Nb5+,21 B3+ + Eu3+ + Nb5+22 co-doped rutile TiO2 ceramics with high dielectric constant and low dielectric loss. Admittedly, the dielectric properties of TiO2 ceramics have been greatly improved in a short time.23,24,25,26 However, with the integration and multifunction of electronic circuits, multifunctional TiO2 ceramics are drawing increasing intention. To the best of our knowledge, there are few works reporting both the nonlinear characteristic and dielectric properties of TiO2 varistors.
It has been confirmed that SiO2 controls the grain size of the varistor while acceptor doping improves the varistor properties of TiO2 ceramic.6,27 Herein, this work reported that (0.30Dy2O3-0.60Nb2O5-0.30SiO2-98.80TiO2) (mol.%) co-doped TiO2 ceramic systems with low breakdown voltage, high nonlinear coefficient and colossal dielectric constant were procured via optimizing sintering temperature and doping concentrations of Dy2O3. The dependence of electrical properties on microstructure was thoroughly investigated and a defect model was proposed to illustrate the effects of Dy2O3 on the properties of the TiO2 varistor.
Experimental Details
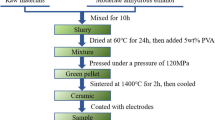
TiO2 ceramic samples (99.10-x)TiO2-xDy2O3-0.60Nb2O5-0.30SiO2 (mol.%, x = 0, 0.15, 0.30, 0.45, 0.60) were prepared by the conventional solid state sintering method. The raw materials TiO2 (99.9%), SiO2 (99.9%), Dy2O3 (99.99%) and Nb2O5 (99.99%) were purchased from Chengdu Chron Chemicals Co., Ltd., China. A total of 0.18 mol chemicals were weighed in stoichiometric proportions and milled in a planetary ball mill for 12 h using 100 mL ethanol as the medium. After drying at 70°C for 18 h, the homogeneous powder mixture was blended with a small amount of PVA (polyvinyl alcohol) solution (PVA/H2O = 8 wt.%). Afterwards, the mixed powder was pressed at 120 MPa into pellets of 15 mm in diameter and 2 mm in thickness. Next, all the pellets were sintered at a designed temperature (1350°C, 1400°C, 1450°C) for 2 h and cooled to room temperature in furnace. To characterize the varistor and dielectric properties of the sintered tablets, both the surfaces were polished and then deposited with silver electrodes, followed by heat treatment at 600°C for 1 h. The varistor properties, including nonlinear coefficient (α) and breakdown voltage (E1mA), were measured by a varistor DC parameter instrument (CJ1001). The dielectric constant, dielectric loss at 1 kHz and the dielectric properties measurements dependent on temperature were conducted over the range of − 150–200°C at a heating rate of 1°C min−1 by an LCR meter (TH2816A). The dielectric properties of samples in the frequency range of 100 Hz–100 kHz were measured by an impedance analyzer (Agilent 4294A) at room temperature. The true density of TiO2 ceramics was estimated by a density meter (ET-320) based on the Archimedes method and the relative density was calculated by the true density and theoretical density accordingly. The crystal structure of TiO2 ceramic samples was determined by an x-ray diffractometer (XRD) (DX-2000) with Cu Kα radiation at a scanning speed of 0.04° s−1 in the 20°–80° 2θ range. After polishing and thermally etching, the surface of TiO2 ceramic samples was observed by scanning electron microscopy (SEM, JSP 7500, Japan). The average grain size is calculated by the intercept method proposed by Mendelson9:
where L is the length of a random line on the SEM image, M is the magnification of the SEM image and N is the number of grain boundaries that the line crosses.
Energy-dispersive x-ray spectroscopy (EDS) was used to analyze the elemental composition and distribution. The valence state of elements in TiO2 samples was tested by x-ray photoelectron spectroscopy (XPS, Kratos AXIS Ultra DLD, Japan).
Results and Discussion
Nonlinear Characteristics
Table I presents the electrical performances of TiO2 ceramics doped with Dy2O3 at different sintering temperatures. When the sintering temperature increases, the nonlinear coefficient and dielectric constant increase, while breakdown voltage and dielectric loss decrease. Altogether, it is observed that the samples possess the best varistor properties after sintering at 1450°C. This phenomenon may be explained by the following equations:
where \( E_{\text{B}} \) is the breakdown voltage, \( N \) is the number of grains per unit length, \( V_{\text{gb}} \) is the voltage barrier at the grain boundary.
According to the above equations, the titanium ion defects can be generated by the dopant of Nb2O5 and the breakdown voltage is determined by the grain size. Increasing the sintering temperature can improve the solubility of Nb2O5, so the ion segregation at the grain boundary is moderated. In this case, more Nb5+ ions replace Ti4+ ions and the resulting ion defects promote the degree of semiconducting of the grains. In addition, the grains grow as the sintering temperature rises, leading to the enhancement of grain boundary barriers and a decrease of grain boundaries per unit thickness.8,12 As a consequence, α increases while \( E_{\text{B}} \) decreases. Throughout this work, we, therefore, picked 1450°C as the sintering temperature for all the Dy2O3-doped samples.
After sintering at 1450°C, the breakdown voltage and nonlinear coefficient of different samples are depicted in Fig. 1a, and the other parameters of samples with different x values are listed in Table II. \( \varPhi_{\text{B}} \) is calculated by the following equations:
where A* is the Richardson constant, K is the Bolzmann constant, T is the absolute temperature, \( \varPhi_{\text{B}} \) is the interface barrier height, and β refers to the relationship illustrated in Eq. 6:
where d is the number of grains per unit length, e is the electron charge (1.602 × 10−19 C), \( \varepsilon_{0} \) is the vacuum dielectric constant (8.85 × 10−14 F/cm), and \( \varepsilon_{\text{r}} \) is the dielectric constant of TiO2 ceramic. Table II and Fig. 1a show that the doping of Dy2O3 obviously affect the properties and microstructure of Nb2O5-SiO2-TiO2 ceramics. Compared to the sample without Dy2O3, all those doped with Dy2O3 possess lower breakdown voltage and higher relative density. The doped Dy2O3 also greatly enhances the dielectric constant without increasing the dielectric loss significantly. In addition, an appropriate doping content of Dy2O3 can improve the nonlinear coefficient of TiO2 ceramics. When the TiO2 varistor is doped with 0.30 mol.% Dy2O3, the breakdown voltage decreases to 1.34 V mm−1, α ascends to 5.7, and the dielectric properties (\( \varepsilon_{\text{r}} \) = 8.01 × 105, \( \tan \delta \) = 0.362) and relative density are optimal (98.22%). However, when doped with excessive Dy2O3 (x > 0.30), the electrical properties of Nb2O5-SiO2-TiO2 ceramics deteriorate. Figure 1b shows that the doping content of Dy2O3 also exerts an evident influence on the J–E characteristics. The curves can be divided into two regions: one is a low-current linear region (J < 0.03 mA cm−2), the other is a nonlinear region.13 The contrast of curves of the Dy2O3-doped samples and the undoped one shows that Dy2O3 can efficiently reduce the breakdown voltage of TiO2 ceramics. In sum, the doping of Dy2O3 remarkably ameliorates the α and \( E_{\text{B}} \) of TiO2 ceramics, while modifying the concentration of Dy2O3 only makes a noticeable contribution to the former. Consequently, Nb2O5-SiO2-TiO2 ceramics doped with 0.30 mol.% Dy2O3 demonstrates the optimum varistor properties.
The classical defect reaction equation can be used to understand how Dy2O3 influences the electrical properties of Nb2O5-SiO2-TiO2 ceramics. To start with, the radius of Dy3+ (0.091 nm) is much larger than that of Ti4+ (0.061 nm), which causes severe lattice distortion and leads to the poor solubility of Dy3+ in TiO2 lattice, thus most of the Dy3+ ions segregate at the grain boundary.8,28 Furthermore, the dissolution of Dy2O3 into TiO2 lattice also induces oxygen vacancies for charge transfer when Dy3+ substitutes for Ti4+, as shown in Eq. 7 below.
When a moderate content of Dy2O3 is doped in the TiO2 varistor, on the one hand, some of the dopant dissolves in the TiO2 lattice to generate oxygen vacancies, and on the other hand, the remaining Dy3+ ions segregate at the grain boundary to form the secondary phase. Consequently, the electrical properties are improved. Nonetheless, if the dopant of Dy2O3 is excessive, more quantities of the secondary phase will be generated by consuming a large number of Dy3+ ions at grain boundary, which decreases the acceptor interface state density at the depletion layer. The final outcome is a decrease in the height of the grain boundary barrier, which can be explained by Eq. 829:
where NS is the acceptor interface state density, \( \varepsilon_{\text{r}} \) is the dielectric constant, \( \varepsilon_{0} \) is the dielectric constant of vacuum and ND is the donor content in the depletion layer. According to Eq. 8, the height of the grain boundary barrier \( \varPhi_{\text{B}} \) is proportional to the acceptor interface state density.
Dielectric Properties
Figure 2 shows the dielectric properties of Nb2O5-Dy2O3-SiO2 co-doped TiO2 ceramics at room temperature in the frequency range of 102–105 Hz. It can be seen from Fig. 2 that the dielectric constant and dielectric loss are nearly steady in the frequency range of 102–103 Hz. However, with the increase of frequency (\( > 10^{3} \) Hz), the dielectric constant decreases while the dielectric loss increases obviously. In addition, when 0.30 mol.% Dy2O3 is added into the TiO2 varistor, the best comprehensive dielectric properties can be obtained at the frequency of 100 Hz, where the dielectric constant is higher than 106 and the dielectric loss is lower than 0.3. Furthermore, in the frequency range of 102–105 Hz, the dielectric constant (> 1 × 105) of the TiO2 samples is much better than the previously reported results.19,21,22,30,31 However, the dielectric loss is still higher than other reports, which makes it necessary to further reduce its dielectric loss for capacitor applications.
Figure 3 shows the dielectric properties of Nb2O5-Dy2O3-SiO2 co-doped TiO2 ceramics at different temperatures measured at 100, 1k, 10k and 100k Hz respectively. It can be seen that the dielectric constant and dielectric loss remain relatively stable over − 150–200°C temperature range at different frequencies, except for an expanded dielectric relaxation around 0°C, which may be caused by electron hopping between Ti4+ and Ti3+.32 In summary, it can be concluded from Figs. 2 and 3 and Table I that 0.30 mol.% Dy2O3-doped TiO2 ceramic possesses the best dielectric properties, with the highest dielectric constant, lower dielectric loss, relatively higher frequency stability.
XRD Analysis
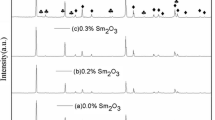
The XRD patterns of TiO2 ceramics doped with different contents of Dy2O3 are exhibited in Fig. 4. Figure 4a demonstrates that only one major phase corresponds to rutile TiO2 (PDF#87-0710) in all the samples. Meanwhile, as the concentration of Dy2O3 increases, the secondary phase Dy2Ti2O7 (PDF#17-0453) appears. Figure 4b is the enlarged partial spectra of Fig. 4a around \( 2\theta = 27.5^{^\circ } \). It can be seen that after Dy2O3 is doped in, the peaks around \( 27.5^{^\circ } \) shift to a larger angle and their full width at half maximum (FWHM) widens, while increasing the content of Dy2O3 dopant makes little difference to the shifting. Basically, the dissolution of Dy3+ in the TiO2 lattice results in severe lattice distortion, thereby decreasing the interplanar spacing value. According to the Bragg formula (9), the angle \( \theta \) shifts to the large-angle direction if the interplanar spacing value \( d \) decreases.15
where λ is the wavelength of the x-ray, \( d \) is the interplanar distance, \( \theta \) is the angle between the incident wave and the crystal plane. Since the solid solubility of Dy3+ in the TiO2 lattice is low, the undissolved Dy3+ ions diffuses to the grain boundary, which is beneficial to the increase of the width of depletion layers and the enhancement of grain boundary potential barrier; therefore, the nonlinear coefficient increases based on the following formula5:
where \( \gamma \) is a constant, E is the external electric field, \( \varPhi_{\text{B}} \) is the potential barrier height. Additionally, the segregation of Dy3+ ions along the grain boundary also produces a secondary phase Dy2Ti2O7.
SEM Analysis
Figure 5 illustrates the SEM images and grain size of different TiO2 ceramic samples. The microstructures show that after Dy2O3 is doped in, the grain size increases, the grain boundary is narrowed and the secondary phase is evenly distributed at the grain boundary initially. Furthermore, as more Dy2O3 is doped, more secondary phase particles are observed. Noticeably, the grain size reaches a maximum of 18.56 μm at x = 0.30 and tends to diminish with the continuous addition of Dy2O3. This phenomenon can be explained as follows: when moderate Dy2O3 is added, oxygen vacancies are generated due to the substitution of Dy3+ ions for Ti4+ ions, which change the oxygen pressure in rutile TiO2.33 Therefore, the dopant of Dy2O3 promotes the growth of grains.8,33 According to Eq. 3, the breakdown voltage (EB) decreases greatly. In addition, the moderate secondary phase can also enhance the dielectric properties due to their high resistance.34 However, the deposition of excessive secondary phase (x > 0.30) at the grain boundary can limit the grain growth. Accordingly, EB increases as a result of the decreasing of grain size. Moreover, by consuming a large number of Dy3+ and Nb5+ ions, the formation of an inordinate secondary phase reduces the acceptor interface state density at the depletion layer, thus causing the nonlinear coefficient to drop according to Eq. 8.
EDS Analysis
To further analyze the elemental distribution and explore the effect of Dy2O3 on the microstructure, the EDS analysis of TiO2 ceramics doped with different contents of Dy2O3 is conducted. Figure 6 shows the point scanning spectra of EDS for samples x = 0 and x = 0.30, respectively, and the corresponding elemental composition is listed in Tables III and IV. Figure 6a and b shows that a small amount Si exists in the grain of both samples. Meanwhile, Fig. 6b and c depicts the difference in elemental composition between the grain and the gain boundary of the 0.30 mol.% Dy2O3-doped sample. Particularly, there exists a very small amount of Dy (~ 0.04%) in the grain, whereas the grain boundary is enriched with a large amount of Dy, Si, Nb in the form of the secondary phase. A reasonable explanation for the phenomenon is that since both radii of Si4+ (0.040 nm) and Dy3+ (0.091 nm) ions differ markedly from the radius of Ti4+ (0.061 nm) ions, the solubility of Si4+ and Dy3+ in TiO2 lattice is low, resulting in the segregation of a large amount of Si4+ and Dy3+ at the grain boundary. Altogether, EDS analysis and the aforementioned XRD detection leads to the conclusion that the stable secondary phase at the grain boundary is Dy2Ti2O7.
Figure 7 shows the elemental mapping of TiO2 ceramics doped with 0 mol.%, 0.30 mol.% as well as 0.60 mol.% Dy2O3 , respectively. It can be seen from Fig. 7a that for the undoped TiO2 varistor, all elements except for Si are evenly distributed in the grain and at the grain boundary, which is consistent with the EDS point analysis. When Dy2O3 is added to the TiO2 varistor, however, the elements Nb, Dy, Si and O are enriched at the grain boundary and the enrichment becomes more obvious with the doping content increasing to 0.60 mol.%. The observation suggests that the doping of Dy2O3 notably changes the elemental distribution of Si and Nb. Normally, the segregated elements are involved in the formation of depletion layers. Nevertheless, there is a secondary phase once the accumulated Nb5+ and Si4+ exceed the saturation value.6,28
Figure 8 depicts the line scanning spectra of EDS for Dy2O3-free and 0.30 mol% Dy2O3-doped TiO2 samples, respectively. As presented in Fig. 8a, the elemental content of Nb and Ti displays no conspicuous variation within the scanning distance, while the content of Si and O fluctuates apparently between the interior and boundary of grain. However, remarkable fluctuations emerge in all the EDS spectra for 0.30 mol% Dy2O3-doped sample at the grain boundary as illustrated in Fig. 8b. These findings are consistent with the results of elemental mapping analysis. Moreover, the grain boundary width can be estimated according to the abrupt variation in the EDS spectra, which turns out to be about 600 nm for the Dy2O3-free sample and approximately 400 nm for the 0.30 mol.% Dy2O3-doped sample, separately. Therefore, the doping of Dy2O3 can also narrow the grain boundary, thereby increasing the density of TiO2 ceramics. In other words, the moderate dopant of Dy2O3 promotes grain growth, narrows the grain boundary and increases the density of TiO2 ceramics, leading to the decrease of grain boundaries per unit thickness. According to Eq. 2, the breakdown voltage decreases.
XPS Analysis
To investigate the corresponding chemical valance states of the TiO2 ceramic sample doped with 0.30 mol.% Dy2O3, XPS analysis is conducted and the results are shown in Fig. 9. It was reported that the dopant of electron-donors such as Nb5+ or Ta5+ generate free electrons in rutile-TiO2 ceramics.17 According to Eqs. 2 and 11, Ti3+ ions is produced by Ti4+ ions capturing free electrons.
Figure 9a shows that two obvious peaks correspond to Ti 2p1/2 and Ti 2p3/2 of BE (binding energy) at 463.5 eV and 457.8 eV, which confirms the presence of Ti4+ ions.35 Furthermore, the other two weak peaks of BE at 458.2 eV and 457.5 eV are attributed to Ti3+.36 Figure 9b shows the O 1 s peaks for the TiO2 sample. It can be seen that three peaks of BE exist at 530.8 eV, 529.5 eV and 529.0 eV. A major peak at 529.0 eV is related to the Ti-O bonds, and the other two peaks at 529.5 eV and 530.8 eV correspond to the existence of other cation-oxygen bonds (i.e., Nb-O, Dy-O and Si-O) and oxygen vacancies or surface hydroxyl, which verifies that some of the dopants (Nb, Dy, Si) enter the lattices and bond with oxygen.16,36 According to Eq. 7, the dopant of acceptor ions like Dy3+ can cause the formation of oxygen vacancies. The defects such as oxygen vacancies and free electrons can increase the conductivity of grains, thus improving the varistor properties of TiO2 ceramics. Meanwhile, Fig. 9c shows two peaks in the BE spectrum of Nb at 209.1 eV as well as 206.3 eV, and the splitting of the spin–orbit is approximately 2.8 eV, which is consistent with the previous report, confirming the existence of Nb5+ ions.16 Figure 9d and e present only a single peak at 152.1 eV and 101.4 eV, respectively, verifying the presence of Dy3+ and Si4+.
Discussion
The dopant of Dy2O3 has a great influence on the microstructure and varistor properties of the Nb-Si co-doped TiO2 ceramics. The model is shown in Fig. 10, where it can been seen that some Ti4+ ions in the lattice are replaced by Dy3+ ions after the addition of Dy2O3, which causes severe lattice distortion and some defect carriers such as oxygen vacancies are generated, thus making the grain more semiconducting.37,38 Therefore, the electrical properties are improved. At the same time, the remaining dopant (Dy2O3) segregates at grain boundary. A moderate content of Dy3+ segregating at the grain boundary improves depletion layer width and increases grain boundary barrier height. As a result, the nonlinear coefficient increases. Moreover, the moderate amount dopant of Dy2O3 also gives rise to the growth of grains. Notably, the secondary phase caused by trivalent ions is helpful to the decrease of dielectric loss.39 According to Eqs. 3 and 10 and the following Eq. 12, the breakdown voltage decreases, the nonlinear coefficient further increases and the dielectric constant ascends.
where \( \varepsilon_{\text{r}} \) is the dielectric constant, \( \varepsilon_{\text{B}} \) is TiO2 intrinsic dielectric constant, \( D \) is the average grain size, \( t_{\text{B}} \) is the mean width of the grain boundary.
However, excessive dopant of Dy2O3 will generate superfluous secondary phase, which inhibit grain growth and degrade the electrical properties. According to the previous report, the IBLC (internal barrier layer capacitance) effect is strengthened as the grain size diminishes.40 Therefore, a colossal dielectric constant can also be achieved in the TiO2 samples containing a mass of secondary phases.40
Conclusion
By optimizing the sintering temperature and the doping content of Dy2O3, good comprehensive varistor properties were obtained in Nb-Dy-Si co-doped TiO2 ceramic systems. The doping of Dy2O3 improves the varistor properties of TiO2 ceramics. When the doping content of Dy2O3 was 0.30 mol.%, the TiO2 ceramic system presented the best varistor properties, with a very low breakdown voltage (1.34 V/mm), good nonlinear coefficient (5.7) and colossal dielectric constant (8.01 × 105) measured at 1 k Hz. Reassuringly, compared to the previous works,41,42 the TiO2 ceramic system in this work has a higher dielectric constant, although the challenge of further reducing its dielectric loss still remains. Table V shows the comparison of electrical properties of different TiO2 varistor samples. In conclusion, the comprehensive varistor properties of the TiO2 ceramic system (99.80TiO2-0.60Nb2O5-0.30Dy2O3-0.30SiO2, mol.%) are better than the previous works, which makes it a potential candidate for low voltage varistors.8,12,14
References
S. Roy, D. Das, and T.K. Roy, J. Alloys Compd. 749, 687 (2018).
J. Shi, Q. Cao, Y. Wei, and Y. Huang, Mater. Sci. Eng., B 99, 344 (2003).
W.Y. Wang, D.F. Zhang, T. Xu, X.F. Li, T. Zhou, and X.L. Chen, J. Alloys Compd. 335, 210 (2002).
S. Marinel, D.H. Choi, R. Heuguet, D. Agrawal, and M. Lanagan, Ceram. Int. 39, 299 (2013).
J. Zhao, B. Wang, and K. Lu, Ceram. Int. 40, 14229 (2014).
J. Li, S. Luo, W. Yao, and Z. Zhang, Mater. Lett. 57, 3748 (2003).
M. Schloffer, C. Teichert, P. Supancic, A. Andreev, Y. Hou, and Z. Wang, J. Eur. Ceram. Soc. 30, 1761 (2010).
F. Peng and D. Zhu, Ceram. Int. 44, 21034 (2018).
E.R.L.V.C. Sousa, J.A. Varela, and E. Longo, J. Eur. Ceram. Soc. 22, 1277 (2002).
J. Zhao, C. Zhang, C. Hu, and K. Lu, J. Eur. Ceram. Soc. 37, 3353 (2017).
F. Meng, Mater. Sci. Eng., B 117, 77 (2005).
J.F.W.C.P. Li, W.B. Su, H.C. Chen, Y.J. Wang, and D.X. Zhuang, Mater. Lett. 57, 1400 (2003).
S.W. Yang, JM. J. Mater. Res. 10, 345 (2011).
S. Luo, Z. Tang, J. Li, and Z. Zhang, Ceram. Int. 34, 1345 (2008).
K. Kang, J. Yan, J. Zhang, J. Du, J. Yi, Y. Liu, R. Bao, S. Tan, and G. Gan, J. Alloys Compd. 649, 1280 (2015).
W. Hu, Y. Liu, R.L. Withers, T.J. Frankcombe, L. Noren, A. Snashall, M. Kitchin, P. Smith, B. Gong, H. Chen, J. Schiemer, F. Brink, and J. Wong-Leung, Nat. Mater. 12, 821 (2013).
W. Tuichai, S. Danwittayakul, N. Chanlek, P. Thongbai, and S. Maensiri, J. Alloys Compd. 703, 139 (2017).
Z. Wang, H. Chen, T. Wang, Y. Xiao, W. Nian, and J. Fan, J. Eur. Ceram. Soc. 38, 3847 (2018).
Y. Yu, W.-L. Li, Y. Zhao, T.-D. Zhang, R.-X. Song, Y.-L. Zhang, Z.-Y. Wang, and W.-D. Fei, J. Eur. Ceram. Soc. 38, 1576 (2018).
W. Wang, L. Li, T. Lu, N. Zhang, and W. Luo, J. Alloys Compd. 806, 89 (2019).
Z. Wang, H. Chen, W. Nian, J. Fan, Y. Li, and X. Wang, J. Alloys Compd. 777, 317 (2019).
X. Guo, Y. Pu, W. Wang, H. Chen, R. Shi, Y. Shi, M. Yang, J. Li, and X. Peng, J. Alloys Compd. 797, 58 (2019).
J. Li, J. Liu, Y. Zhuang, Y. Tian, Q. Hu, Z. Xu, L. Wang, and F. Li, J. Alloys Compd. 786, 377 (2019).
J. Fan, S. Leng, Z. Cao, W. He, Y. Gao, J. Liu, and G. Li, Ceram. Int. 45, 1001 (2019).
S.D.W. Tuichaia, N. Chanlekc, and P. Thongbaia, Mater. Res. Bull. 116, 137 (2019).
P.L.H. Peng, D. Wu, X. Zhou, Z. Peng, Y. Xiang, X. Chao, and Z. Yang, J. Alloys Compd. 783, 423 (2019).
L. Meng, G. Li, L. Zheng, L. Cheng, J. Zeng, H. Huang, and S. Bernik, J. Am. Ceram. Soc. 94, 2300 (2011).
Q. Wang, G. Lian, and E.C. Dickey, Acta Mater. 52, 809 (2004).
H. Bai, M. Li, Z. Xu, R. Chu, J. Hao, H. Li, C. Chen, and G. Li, J. Eur. Ceram. Soc. 37, 3965 (2017).
T. Nachaithong, P. Thongbai, and S. Maensiri, J. Eur. Ceram. Soc. 37, 655 (2017).
T. Nachaithong, W. Tuichai, P. Kidkhunthod, N. Chanlek, P. Thongbai, and S. Maensiri, J. Eur. Ceram. Soc. 37, 3521 (2017).
N.Z.C. Wang, Q. Li, Y. Yu, J. Zhang, Y. Li, and H. Wang, J. Am. Ceram. Soc. 98, 148 (2015).
Z.-L.M. Tang, Z.-T. Zhang, and Z.-G. Zhou, Acta Phys.-Chim. Sin. 21, 1122 (2005).
E. Swatsitang, K. Prompa, and T. Putjuso, J. Alloys Compd. 789, 231 (2019).
G. Tan, W. Liu, X. Xue, and H. Hao, J. Alloys Compd. 617, 265 (2014).
X. Cheng, Z. Li, and J. Wu, J. Mater. Chem. A 3, 5805 (2015).
J. Li, F. Li, Y. Zhuang, L. Jin, L. Wang, X. Wei, Z. Xu, and S. Zhang, J. Appl. Phys. 116, 074105 (2014).
L. Li, T. Lu, N. Zhang, J. Li, and Z. Cai, J. Mater. Chem. C 6, 2283 (2018).
C. Zhao, and J. Wu, ACS Appl. Mater. Int. 10, 3680 (2018).
Z. Li, J. Wu, and C. Zhao, J. Mater. Chem. C 7, 4235 (2019).
X. Wei, W. Jie, Z. Yang, F. Zheng, H. Zeng, Y. Liu, and J. Hao, J. Mater. Chem. C 3, 11005 (2015).
C. Yang, M.-Y. Tse, X. Wei, and J. Hao, J. Mater. Chem. C 5, 5170 (2017).
Acknowledgments
The authors appreciate Wang Hui from the Analytical & Testing Center of Sichuan University for her help with SEM and EDS characterization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liao, X., Peng, F., Pu, Y. et al. Effects of Dy2O3 on the Electrical Properties of a (Nb2O5-Dy2O3-SiO2) Co-doped TiO2 Varistor. J. Electron. Mater. 50, 1963–1979 (2021). https://doi.org/10.1007/s11664-020-08727-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-020-08727-1