Abstract
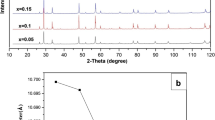
In this study, Ruddlesden–Popper La0.9Sr1.1Co1−xMoxO4 (x ≤ 0.1) powders were successfully synthesized using a modified sol–gel method. The structural analysis revealed that all samples had a tetragonal phase at room temperature. The electrical conductivity measurements showed that all samples had semiconducting behavior in the range of room temperature to 850°C. Moreover, it was found that the electrical conductivity of the ceramics was enhanced with the increase of Mo doping, at temperatures higher than 300°C up to 850°C. This enhancement of the electrical conductivity can be due to Co3+–O–Mo5+–O–Co2+ double-exchange interaction. The EIS measurements of the symmetrical cells were carried out for x = 0, 0.03 and 0.1 samples at 650°C, 750°C and 850°C. The obtained area specific resistance (ASR) values of La0.9Sr1.1Co1−xMoxO4 on ceria-gadolinium oxide (CGO) electrolyte at 850°C were 0.36 Ω cm2, 0.35 Ω cm2 and 1 Ω cm2 for samples x = 0, 0.03 and 0.1, respectively. These results indicate that the electrical conductivity of pure sample (La0.9Sr1.1CoO4) has been enhanced by Mo substitution in Co ion sites, but limits the oxygen ion transport.
Similar content being viewed by others
References
Y. Wang, J. Cheng, Q. Jiang, J. Yang, and J. Gao, J. Power Sour. 196, 3104 (2011).
D.J. Brett, A. Atkinson, N.P. Brandon, and S.J. Skinner, Chem. Soc. Rev. 37, 1568 (2008).
A. Gómez-Pérez, M.T. Azcondo, M. Yuste, J.C. Pérez-Flores, N. Bonanos, F. Porcher, A. Muñoz-Noval, M. Hoelzel, F. García-Alvarado, and U. Amador, J. Mater. Chem. A 4, 3386 (2016).
Z. Zhang, Y. Zhu, Y. Zhong, W. Zhou, and Z. Shao, Adv. Energy Mater. 7, 1700242 (2017).
J. Cui, Y. Gong, R. Shao, S. Wang, J. Mao, M. Yang, W. Wang, and Q. Zhou, J. Mater. Sci.: Mater. Electron. 30, 5573 (2019).
I. Belenkaya, A. Matvienko, and A. Nemudry, J. Mater. Chem. A 3, 23240 (2015).
I. Belenkaya, A. Matvienko, and A. Nemudry, J. Appl. Crystallogr. 48, 179 (2015).
M. Popov, I. Starkov, S. Bychkov, and A. Nemudry, J. Membr. Sci 469, 88 (2014).
O. Savinskaya and A. Nemudry, J. Membr. Sci 459, 45 (2014).
S. Huang, S. Feng, Q. Lu, Y. Li, H. Wang, and C. Wang, J. Power Sour. 251, 357 (2014).
A. Aguadero, D. Pérez-Coll, J. Alonso, S. Skinner, and J. Kilner, Chem. Mater. 24, 2655 (2012).
J. Wang, T. Yang, L. Lei, and K. Huang, J. Mater. Chem. A 5, 8989 (2017).
V. Cascos and J. Alonso, Materials 9, 579 (2016).
V. Cascos, R. Martínez-Coronado, and J. Alonso, Int. J. Hydrog. Energy 39, 14349 (2014).
M. Li, M. Zhao, F. Li, W. Zhou, V.K. Peterson, X. Xu, Z. Shao, I. Gentle, and Z. Zhu, Nat. Commun. 8, 13990 (2017).
A. Demont, R. Sayers, M.A. Tsiamtsouri, S. Romani, P.A. Chater, H. Niu, C. Martí Gastaldo, Z. Xu, Z. Deng, and Y. Bréard, J. Am. Chem. Soc 135, 10114 (2013).
E. Shubnikova, O. Bragina, and A. Nemudry, J. Ind. Eng. Chem. 59, 242 (2018).
R. Martínez-Coronado, J. Alonso, and M. Fernández-Díaz, J. Power Sour. 258, 76 (2014).
S. Huang, Q. Lu, S. Feng, G. Li, and C. Wang, Adv. Energy Mater 1, 1094 (2011).
Y.-F. Sun, Y.-Q. Zhang, B. Hua, Y. Behnamian, J. Li, S.-H. Cui, J.-H. Li, and J.-L. Luo, J. Power Sour. 301, 237 (2016).
X. Li and Y. Li, J. Mol. Catal. A Chem. 386, 69 (2014).
T. Wei, Q. Zhang, Y.-H. Huang, and J.B. Goodenough, J. Mater. Chem. 22, 225 (2012).
T. Ghorbani-Moghadam, A. Kompany, M. Bagheri-Mohagheghi, and M.E. Abrishami, J. Magn. Mag. Mater 465, 768 (2018).
Z. Talaei, H. Salamati, and A. Pakzad, Int. J. Hydrog. Energy 35, 9401 (2010).
A. Pakzad, H. Salamati, P. Kameli, and Z. Talaei, Int. J. Hydrog. Energy 35, 9398 (2010).
S. Gómez, J. Gurauskis, V. Øygarden, D. Hotza, T. Grande, and K. Wiik, Solid State Ion 292, 38 (2016).
W. Lu, Y. Sun, R. Ang, X. Zhu, and W. Song, Phys. Rev. B 75, 014414 (2007).
M.V. Sandoval, C. Pirovano, E. Capoen, R. Jooris, F. Porcher, P. Roussel, and G.H. Gauthier, Int. J. Hydrog. Energy 42, 21930 (2017).
Y.S. Chung, T. Kim, T.H. Shin, H. Yoon, S. Park, N.M. Sammes, W.B. Kim, and J.S. Chung, J. Mater. Chem. A 5, 6437 (2017).
Y.-P. Wang, Q. Xu, D.-P. Huang, K. Zhao, M. Chen, and B.-H. Kim, Int. J. Hydrog. Energy 42, 6290 (2017).
L.V. Makhnach, V.V. Pankov, and P. Strobel, Mater. Chem. Phys. 111, 125 (2008).
P.I. Cowin, R. Lan, C.T. Petit, and S. Tao, Mater. Chem. Phys. 168, 50 (2015).
M. Itoh, I. Ohta, and Y. Inaguma, Mater. Sci. Eng. B 41, 55 (1996).
F. Riza and C. Ftikos, J. Eur. Ceram. Soc. 27, 571 (2007).
M. Stawarz and P.M. Nuckowski, Materials 13, 1745 (2020).
E.P. Murray and S.A. Barnett, Solid State Ion. 143(3--4), 265 (2001).
Q. Li, H. Zhao, L. Huo, L. Sun, X. Cheng, and J.-C. Grenier, Electrochem. Commun. 9, 1508 (2007).
S. Liping, H. Lihua, Z. Hui, L. Qiang, and C. Pijolat, J. Power Sour. 179, 96 (2008).
Y. Hu, Y. Bouffanais, L. Almar, A. Morata, A. Tarancon, and G. Dezanneau, Int. J. Hydrog. Energy 38, 3064 (2013).
M. Rieu, R. Sayers, M. Laguna-Bercero, S. Skinner, P. Lenormand, and F. Ansart, J. Electrochem. Soc. 157, B477 (2010).
Acknowledgments
This work has been financially supported by the Vice President for Research and Technology, Ferdowsi University of Mashhad, Iran, under Grant No. 3-40687.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghorbani-Moghadam, T., Kompany, A., Bagheri-Mohagheghi, M.M. et al. Characterization, Electrical and Electrochemical Study of La0.9Sr1.1Co1−xMoxO4 (x ≤ 0.1) as Cathode for Solid Oxide Fuel Cells. J. Electron. Mater. 49, 6448–6454 (2020). https://doi.org/10.1007/s11664-020-08404-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-020-08404-3