Abstract
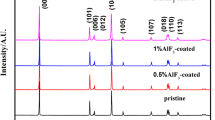
A uniform nanocoating can substantially enhance the electrochemical properties of cathode materials. Herein, we report that a uniform AlPO4 coating can be produced on the surface of LiNi0.8Co0.15Al0.05O2 (NCA) particles by a homogeneous precipitation method, which was confirmed by scanning electron microscopy and energy dispersive x-ray spectroscopy. After being heated, the AlPO4 was converted to the Li3PO4 which was verified by x-ray diffraction. The heated AlPO4 coated NCA demonstrated a substantially enhanced electrochemical performance. The capacity retention increased from 87.38% for bare NCA to 94.28% for the heated 1 wt.% AlPO4 coated NCA sample. Moreover, the reversible capacity at 5 C increased from 80 mAh/g for bare NCA to 120 mAh/g for the heated 1 wt.% AlPO4-coated NCA. In addition, improved thermal stability was also found. The start temperature of thermal runaway increased from 175.12°C for bare NCA to 200.32°C for the heated 1 wt.% AlPO4-coated NCA.
Similar content being viewed by others
References
J.W. Choi and D. Aurbach, Nat. Rev. Mater. 1, 16013 (2016).
J.B. Goodenough and Y. Kim, Chem. Mater. 22, 587 (2010).
C.P. Grey and J.M. Tarascon, Nat. Mater. 16, 45 (2016).
P.Y. Hou, L.Q. Zhang, and X.P. Gao, J. Mater. Chem. A 2, 17130 (2014).
P.K. Nayak, E. Levi, J. Grinblat, M. Levi, B. Markovsky, N. Munichandraiah, Y.K. Sun, and D. Aurbach, Chemsuschem 9, 2404 (2016).
N. Nitta, F. Wu, J.T. Lee, and G. Yushin, Mater. Today 18, 252 (2015).
R. Robert, C. Villevieille, and P. Novák, J. Mater. Chem. A 2, 8589 (2014).
J. Kim, H. Lee, H. Cha, M. Yoon, M. Park, and J. Cho, Adv. Energy Mater. 8, 1870023 (2018).
S.-T. Myung, F. Maglia, K.-J. Park, C.S. Yoon, P. Lamp, S.-J. Kim, and Y.-K. Sun, ACS Energy Lett. 2, 196 (2016).
Y. Xia, J. Zheng, C. Wang, and M. Gu, Nano Energy 49, 434 (2018).
E. Cho, S.W. Seo, and K. Min, ACS Appl. Mater. Interfaces 9, 33257 (2017).
P. Hou, H. Zhang, X. Deng, X. Xu, and L. Zhang, ACS Appl. Mater. Interfaces 9, 29643 (2017).
E. Jo, S. Hwang, S.M. Kim, and W. Chang, Chem. Mater. 29, 2708 (2017).
F. Schipper, E.M. Erickson, C. Erk, J.-Y. Shin, F.F. Chesneau, and D. Aurbach, J. Electrochem. Soc. 164, A6220 (2016).
T. Chen, X. Li, H. Wang, X. Yan, L. Wang, B. Deng, W. Ge, and M. Qu, J. Power Sour. 374, 1 (2018).
Y.-M. Chung, S.-H. Ryu, J.-H. Ju, Y.-R. Bak, M.-J. Hwang, K.-W. Kim, K.-K. Cho, and K.-S. Ryu, Bull. Korean Chem. Soc. 31, 2304 (2010).
S. Yoon, K.-N. Jung, S.-H. Yeon, C.S. Jin, and K.-H. Shin, J. Electroanal. Chem. 683, 88 (2012).
G. Dai, M. Yu, F. Shen, J. Cao, L. Ni, Y. Chen, Y. Tang, and Y. Chen, Ionics 22, 2021 (2016).
B. Han, B. Key, S.H. Lapidus, J.C. Garcia, H. Iddir, J.T. Vaughey, and F. Dogan, ACS Appl. Mater. Interfaces 9, 41291 (2017).
H.B. Kim, B.C. Park, S.T. Myung, K. Amine, J. Prakash, and Y.K. Sun, J. Power Sour 179, 347 (2008).
R. Qi, J.-L. Shi, X.-D. Zhang, X.-X. Zeng, Y.-X. Yin, J. Xu, L. Chen, W.-G. Fu, Y.-G. Guo, and L.-J. Wan, Sci China Chem 60, 1230 (2017).
G. Wu and Y. Zhou, J. Energy Chem. 28, 151 (2018).
K. Min, S.W. Seo, B. Choi, K. Park, and E. Cho, ACS Appl. Mater. Interfaces 9, 17822 (2017).
S. Chen, T. He, Y. Su, Y. Lu, L. Bao, L. Chen, Q. Zhang, J. Wang, R. Chen, and F. Wu, ACS Appl. Mater. Interfaces 9, 29732 (2017).
J. Cho, Y.W. Kim, B. Kim, J.G. Lee, and B. Park, Angew. Chem. Int. Ed. Engl. 42, 1618 (2003).
D. Chen, F. Zheng, L. Li, M. Chen, X. Zhong, W. Li, and L. Lu, J. Power Sour. 341, 147 (2017).
Z.-F. Tang, R. Wu, P.-F. Huang, Q.-S. Wang, and C.-H. Chen, J. Alloys Compd. 693, 1157 (2017).
P. Hou, H. Zhang, Z. Zi, L. Zhang, and X. Xu, J. Mater. Chem. A 5, 4254 (2017).
F.L. Yang, W. Zhang, Z.X. Chi, F.Q. Cheng, J.T. Chen, A.M. Cao, and L.J. Wan, Chem. Commun. (Camb.) 51, 2943 (2015).
H. Dong, S. Li, H. Liu, J. Mei, H. Liu, and G. Liu, Ionics 25, 827 (2019).
Y. Zhou, Y. Wang, S. Li, J. Mei, H. Liu, H. Liu, and G. Liu, J. Alloys Compd. 695, 2951 (2017).
A.T.M. Appapillai, A.N. Mansour, J. Cho, and Y. Shao-Horn, Chem. Mater. 19, 10 (2007).
F. Wu, X. Zhang, T. Zhao, L. Li, M. Xie, and R. Chen, ACS Appl. Mater. Interfaces 7, 3773 (2015).
Y. Wu, A.V. Murugan, and A. Manthiram, J. Electrochem. Soc. 155, A635 (2008).
G.-R. Hu, X.-R. Deng, Z.-D. Peng, and K. Du, Electrochim. Acta 53, 2567 (2008).
Z.W. Lebens-Higgins, S. Sallis, N.V. Faenza, F. Badway, N. Pereira, D.M. Halat, M. Wahila, C. Schlueter, T.-L. Lee, W. Yang, C.P. Grey, G.G. Amatucci, and L.F.J. Piper, Chem. Mater. 30, 958 (2018).
L. Liang, X. Sun, C. Wu, L. Hou, J. Sun, X. Zhang, and C. Yuan, ACS Appl. Mater. Interfaces 10, 5498 (2018).
Y.K. Hu, J.X. Ren, Q.L. Wei, X.D. Guo, Y. Tang, B.H. Zhong, and H. Liu, Acta Phys. Chim. Sin. 30, 75 (2014).
J. Cho, T.-G. Kim, C. Kim, J.-G. Lee, Y.-W. Kim, and B. Park, J. Power Sour. 146, 58 (2005).
L. Li, Y. Cao, H. Zheng, and C. Feng, J. Mater. Sci. Mater. Electron. 28, 1925 (2016).
X. Ma, C. Wang, X. Han, and J. Sun, J. Alloys Compd. 453, 352 (2008).
Y. Zeng and J. He, J. Power Sour. 189, 519 (2009).
Y. Chen, K. Xie, C. Zheng, Z. Ma, and Z. Chen, ACS Appl. Mater. Interfaces 6, 16888 (2014).
B. Qiu, J. Wang, Y. Xia, Z. Wei, S. Han, and Z. Liu, ACS Appl. Mater. Interfaces 6, 9185 (2014).
L. Xiong, Y. Xu, T. Tao, and J.B. Goodenough, J. Power Sour. 199, 214 (2012).
J.M. Zheng, X.B. Wu, and Y. Yang, Electrochim. Acta 56, 3071 (2011).
Acknowledgments
The research was financially supported by the Sichuan Provincial Key Technology R&D Program (2016GZ0299).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Liu, G., Li, S. et al. Preparation of a Homogeneous Li3PO4 Coating and Its Effect on the Electrochemical Properties of LiNi0.8Co0.15Al0.05O2. J. Electron. Mater. 48, 4443–4451 (2019). https://doi.org/10.1007/s11664-019-07223-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-019-07223-5