Abstract
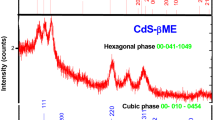
A facile and simple synthetic route has been employed to synthesize rod-shaped optically efficient cadmium sulfide (CdS) mesoscopic structures using high concentrations of cetyl trimethyl ammonium bromide (CTAB) as the stabilizing agent. The mesoscopic structures were characterized using x-ray diffaractometer (XRD), scanning electron microscopy, UV–visible, photoluminescence (PL), and Fourier transform and infrared (FTIR) spectroscopy. It was found that, if the concentration of CTAB is significantly higher than its critical micelle concentration, the nucleation of CdS mesoscopic structures resulted in rod-like structures. The size of the mesoscopic structures initially increased and then decreased with band gaps 2.5–2.7 eV. XRD analysis showed that the samples had a pure cubic phase confirming the particle size. The values of Urbach energy for the absorption tail states were determined and found to be in agreement with the single crystal. PL spectra showed sharp green emission peaks in the 530-nm to 560-nm wavelength range. FTIR spectra showed the adsorption mode of CTAB onto the CdS mesoscopic structures. A possible mechanism of formation of rod-shaped CdS mesoscopic structures is also elucidated.
Similar content being viewed by others
References
C. Zhou, Y. Geng, Q. Chen, J. Xu, N. Huang, Y. Gan, and L. Zhou, Mater. Lett. 172, 171 (2016).
C.V. Gopi, M. Venkata-Haritha, S. Kim, and H.J. Kim, RSC Adv. 5, 2963 (2015).
Y. Mo, Y. Tang, F. Gao, J. Yang, and Y. Zhang, Ind. Eng. Chem. Res. 51, 5995 (2012).
G. Konstantatos, I. Howard, A. Fischer, S. Hoogland, J. Clifford, E. Klem, L. Levina, and E.H. Sargent, Nature 442, 180 (2006).
Y. Guo, L. Jiang, L. Wang, X. Shi, Q. Fang, L. Yang, and C. Shan, Mater. Lett. 74, 26 (2012).
T. Shanmugapriya, R. Vinayakan, K.G. Thomas, and P. Ramamurthy, CrystEngComm 13, 2340 (2011).
Y. Zou, D. Li, and D. Yang, Nanoscale Res. Lett. 6, 374 (2011).
K.D. Nisha, M. Navaneethan, Y. Hayakawa, S. Ponnusamy, and C. Muthamizhchelvan, Mater. Chem. Phys. 136, 1038 (2012).
W. Zhang, H. Zhang, and X. Zhong, RSC Adv. 3, 17477 (2013).
S. Mridha and D. Basak, Phys. Status Solidi A 206, 1515 (2009).
D.N. Rubingh and P.M. Holland, Surfactant science series (New York: M. Dekker, 1991), p. 525.
A.B. Wong, S. Brittman, Y. Yu, N.P. Dasgupta, and P. Yang, Nano Lett. 15, 4096 (2015).
K. Wu, Y. Du, H. Tang, Z. Chen, and T. Lian, J. Am. Chem. Soc. 137, 10224 (2015).
R. Narayanan, M. Deepa, and A.K. Srivastava, Phys. Chem. Chem. Phys. 14, 767 (2012).
I.H. Arellano, J. Mangadlao, I.B. Ramiro, and K.F. Suazo, Mater. Lett. 64, 785 (2010).
S. Kumar, M. Gradzielski, and S.K. Mehta, RSC Adv. 3, 2662 (2013).
G. Pandey and S. Dixit, J. Phys. Chem. C 115, 17633 (2011).
N. Gonçalves, J. Carvalho, Z. Lima, and J. Sasaki, Mater. Lett. 72, 36 (2012).
B. Pejova, Mater. Chem. Phys. 119, 367 (2010).
A. Podborska, B. Gaweł, L. Pietrzak, I.B. Szymańska, J.K. Jeszka, W. łasocha, and K. Szaciłowski, J. Phys. Chem. C 113, 6774 (2009).
A.E. Rakhshani, J. Phys. Condens. Matter 12, 4391 (2000).
V. Singh and P. Chauhan, J. Phys. Chem. Solids 70, 1074 (2009).
Z. Lin, J.J. Cai, L.E. Scriven, and H.T. Davis, J. Phys. Chem. 98, 5984 (1994).
Z. Wang and R.G. Larson, J. Phys. Chem. B 113, 13697 (2009).
N. Vlachy, M. Drechsler, J. Verbavatz, D. Touraud, and W. Kunz, J. Colloid Interface Sci. 319, 542 (2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aslam, S., Mustafa, F., Jamil, A. et al. Efficient Tuning of Optical Properties and Morphology of Mesoscopic CdS via a Facile Route. J. Electron. Mater. 47, 3701–3708 (2018). https://doi.org/10.1007/s11664-018-6225-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-018-6225-6