Abstract
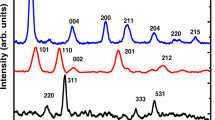
Aqueous industrial wastes from heavy industry factories contain a large amount of Fe ions, which constitute a hazard for human life even at trace concentrations. Adsorption technology is a promising method for removing Fe(III) from aqueous solutions. In this report, the adsorption of the Fe(III) ion on γ- and α-MnO2 nanostructures was compared. The results showed that the maximum adsorption was obtained at pH = 3.5 for both materials after 120 min for γ-MnO2 and 80 min for α-MnO2. Adsorption isotherm models, such as Langmuir, Freundlich, Sips, Tempkin, and Dubinin–Radushkevich were applied to determine adsorption capacity as well as the nature of the uptake. The highest R 2, the smallest of root mean squared error (RMSE), and the nonlinear Chi-square test (χ2) values determined that the Sips model was the most appropriate equation to describe the adsorption of Fe(III) on γ- and α-MnO2. The maximum monolayer adsorption capacity calculated from the Langmuir model and the maximum adsorption capacity calculated from the Sips model of γ-MnO2 was more than four times that of α-MnO2. The heat of the adsorption as well as the mean free energy estimated from Tempkin and Dubinin–Radushkevich was determined to be less than 8 kJ/mol, which showed that the adsorption on both materials followed a physical process. Kinetic studies showed that a pseudo-second-order model was accurately described on both samples with three stages.
Similar content being viewed by others
References
H.A. Talaat, M.Y. Ghaly, E.M. Kamel, E.M. Ahmed, and A.M. Awad, J. Am. Sci. 6, 7 (2010).
G. Hodaifa, J.M. Ochando-Pulido, S.B. Driss Alami, S. Rodriguez-Vives, and A. Martinez-Ferez, Ind. Crop. Prod. 49, 9 (2013).
L.M. Nieto, S.B.D. Alami, G. Hodaifa, C. Faur, S. Rodríguez, J.A. Giménez, and J. Ochando, Ind. Crop. Prod. 32, 5 (2010).
T.M. Alslaibi, I. Abustan, M.A. Ahmad, and A.A. Foul, Desalin. Water Treat. 52, 11 (2014).
A. Burke, E. Yilmaz, N. Hasirci, and O. Yilmaz, J. Appl. Polym. Sci. 84, 8 (2002).
U. Kouakou, A.S. Ello, J.A. Yapo, and A. Trokourey, J. Environ. Chem. Ecotoxicol. 5, 4 (2013).
M.K. Seliem and S. Komarneni, Microporous. Mesoporous. Mater. 228, 9 (2016).
M. Sun, B. Lan, L. Yu, F. Ye, W. Song, J. He, G. Diao, and Y. Zheng, Mater. Lett. 86, 3 (2012).
F. Tedjar and J. Guitton, Thermochim. Acta. 181, 10 (1991).
M. Singh, D.N. Thanh, P. Ulbrich, N. Strnadová, and F. Štěpánek, J. Solid State Chem. 183, 8 (2010).
L. Ngoc Chung and D. Van Phuc, Adv. Nat. Sci.: Nanosci. Nanotechnol. 6, 8 (2015).
V.-P. Dinh, N.-C. Le, T.-P.-T. Nguyen, and N.-T. Nguyen, J. Chem. 2016, 9 (2016).
K.Y. Foo and B.H. Hameed, Chem. Eng. J. 156, 9 (2010).
J. Anwar, U. Shafique, Z. Waheed uz, M. Salman, A. Dar, and S. Anwar, Bioresour. Technol. 101, 4 (2010).
R.R. Bhatt and B.A. Shah, Arab J. Chem. 8, 13 (2015).
F.-C. Wu, R.-L. Tseng, and R.-S. Juang, J. Hazard. Mater. 73, 13 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dinh, VP., Le, NC., Le, TD. et al. Comparison of the Adsorption of Fe(III) on Alpha- and Gamma-MnO2 Nanostructure. J. Electron. Mater. 46, 3681–3688 (2017). https://doi.org/10.1007/s11664-017-5287-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-017-5287-1