Abstract
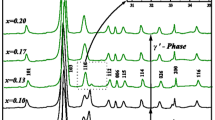
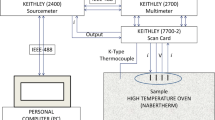
The solid electrolyte is one of the most important components for a solid oxide fuel cell (SOFC). The various divalent or trivalent metal ion-doped bismuth-based materials exhibit good ionic conductivity. Therefore, these materials are used as electrolytes in the SOFC. In this paper, the samples of (Bi0.92−x Ho0.03Er0.05)2O3 + (ZnO) x solutions with a 0 ≤ x ≤ 0.2 molar ratio are synthesized by the solid state reaction method. The detailed structural and electrical characterizations are investigated by using x-ray diffraction (XRD), alternating current electrochemical impedance spectroscopy, and scanning electron microscopy (SEM). The XRD patterns of all samples are indexed on a monoclinic symmetry with a P21/c space group. In addition, the rietveld parameters are determined by using the FullProf software program. The impedance measurements of the samples are obtained at the 1 Hz to 20 MHz frequency range. The impedance value of the pellets increases with temperature. Based on the impedance results, it is found that the contribution of grain (bulk) is more than a grain boundary in terms of conductivity, which permits the attribution of a grain boundary. The ionic conductivity decreases with an increasing amount of Zn contribution. The value of highest electrical conductivity among all samples is calculated as 0.358 S cm−1 at 800°C for undoped (Bi0.92Ho0.03Er0.05)2O3.
Similar content being viewed by others
References
P. Satardekar, D. Montinaro, and V.M. Sglavo, Ceram. Int. 41, 9806 (2015).
T.C. Kuo, Y.L. Kuo, and W.C.J. Wei, J. Eur. Ceram. Soc. 31, 3153 (2011).
H. Ozlu Torun, S. Cakar, E. Ersoy, and O. Turkoglu, J. Therm. Anal. Calorim. 122, 525 (2015).
A. Watanabe and M. Sekita, Solid State Ion. 176, 2429 (2005).
T. Chou, L. Der Liu, and W.C.J. Wei, J. Eur. Ceram. Soc. 31, 3087 (2011).
N. Jiang, E.D. Wachsman, and S.H. Jung, Solid State Ion. 150, 347 (2002).
B.F.D. Mercurio and M. El Farissi, Solid State Ion. 39, 297 (1990).
G. Meng, C. Chen, X. Han, P. Yang, and D. Peng, Solid State Ion. 28–30, 533 (1988).
N. Portefaix, P. Conflant, J.C. Boivin, J.P. Wignacourt, and M. Drache, J. Solid State Chem. 226, 219 (1997)
C.Y. Chen, J.C. Weng, J.H. Chen, S.H. Ma, K.H. Chen, T.L. Horng, C.Y. Tsay, C.J. Chang, C.K. Lin, and J.J. Wu, Powder Technol. 272, 316 (2015).
Y.Z. Haiping, J. Zhang, and J. Wang, J. Rare Earths 24, 408 (2006).
P. Shuk, Solid State Ion. 89, 179 (1996).
M.J. Verkerk and A.J. Burggraaf, J. Electrochem. Soc. 128, 75 (1981).
A. Watanabe, Solid State Ion. 3, 35 (1989).
S.F. Wang, Y.F. Hsu, W.C. Tsai, and H.C. Lu, J. Power Sources 218, 106 (2012).
N.A.S. Webster, C.D. Ling, C.L. Raston, and F.J. Lincoln, Solid State Ion. 179, 697 (2008).
V. Fruth, A. Ianculescu, D. Berger, S. Preda, G. Voicu, E. Tenea, and M. Popa, J. Eur. Ceram. Soc. 26, 3011 (2006).
J. Huang, F. Xie, C. Wang, and Z. Mao, Int. J. Hydrogen Energy 37, 877 (2012).
G. Malmros, L. Fernholt, C.J. Ballhausen, U. Ragnarsson, S.E. Rasmussen, E. Sunde, and N.A. Sørensen, Acta Chem. Scand. 24, 384 (1970).
K.E.S. AronWalsh, G.W. Watson, D.J. Payne, R.G. Edgell, J. Guo, P.A. Glans, and T. Learmonth, Phys. Rev. B 73, 235104 (2006).
Y.Y.Y. Li and F. Yang, Appl. Surf. Sci. 358, 449 (2015).
H.C. Wang, C.L. Wang, W.B. Su, J. Liu, Y. Zhao, H. Peng, J.L. Zhang, M.L. Zhao, J.C. Li, N. Yin, and L.M. Mei, Mater. Res. Bull. 45, 809 (2010).
Y.Z.Y. Wang, J. Zhao, B. Zhou, X. Zhao, and Z. Wang, J. Alloys Compd. 592, 296 (2014).
R.S. Levin and E.M. Roth, J. Res. Natl. Bur. Stand. 68, 189 (1964).
P.M. Saffronov, G.M. Batog, V.N. Stepayuk, and T.V. Federov, Russ. J. Inorg. Chem. 16, 460 (1971).
W.G. Wong and J. Morris, Ceram. Bull. 53, 816 (1974).
D. Suvorov, J. Guha, and S. Kunej, J. Mater. Sci. 39, 911 (2004).
J. Luo, R.J. Ball, and R. Stevens, J. Mater. Sci. 39, 235 (2004).
J.E. Bauerle, J. Phys. Chem. Solids 30, 2657 (1969).
A. Arabaci, Ceram. Int. 41, 5836 (2015).
S. Le, J. Zhang, X. Zhu, J. Zhai, and K. Sun, J. Power Sources 232, 219 (2013).
S. Gupta and K. Singh, Solid State Ion. 278, 233 (2015).
B. Aktas, S. Tekeli, and M. Kucuktuvek, J. Mater. Eng. Perform. 23, 349 (2014).
R. Kant, K. Singh, and O. Pandey, Int. J. Hydrogen Energy 33, 455 (2008).
S. Sanna, V. Esposito, C. Graves, J. Hjelm, J.W. Andreasen, and N. Pryds, Solid State Ion. 266, 13 (2014).
N.M. Sammes, G.A. Tompsett, H. Näfe, and F. Aldinger, J. Eur. Ceram. Soc. 19, 1801 (1999).
Acknowledgements
The authors acknowledge the support provided by the Research and Application Center for Hydrogen Technologies, Suleyman Demirel University, Turkey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ermiş, İ., Çorumlu, V., Sertkol, M. et al. Microstructure and Electrical Conductivity of ZnO Addition on the Properties of (Bi0.92Ho0.03Er0.05)2O3 . J. Electron. Mater. 45, 5860–5866 (2016). https://doi.org/10.1007/s11664-016-4799-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-016-4799-4